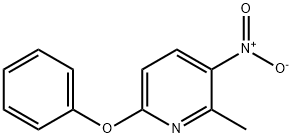
2-Methyl-3-Nitro-6-Phenoxypyridine synthesis
- Product Name:2-Methyl-3-Nitro-6-Phenoxypyridine
- CAS Number:28232-35-1
- Molecular formula:C12H10N2O3
- Molecular Weight:230.22
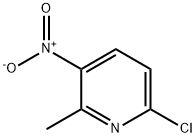
22280-60-0
315 suppliers
$10.00/1g

108-95-2
726 suppliers
$14.00/25g

28232-35-1
15 suppliers
inquiry
Yield:28232-35-1 97%
Reaction Conditions:
with caesium carbonate in acetonitrile at 0 - 20; for 15 h;
Steps:
A 2-methyl-3-nitro-6-phenoxypyridine
A round bottom flask containing 6-chloro-2- methyl-3-nitropyridine (50.1 g, 290 mmol) and CH3CN (230 mL) was cooled at 0 °C. Phenol (41.0 g, 436 mmol) was added followed by Cs2C03 (148 g, 454 mmol). The reaction mixture was allowed to warm to room temperature and stirred for 15 h. The mixture was transferred to a 2L Erlenmeyer flask, then diluted with water to a total volume of 1.8 L. The resulting suspension was stirred at room temperature for 10 min, then the solid was isolated by filtration, rinsed with water and dried to yield the title compound (64.7 g, 97% yield) as a brown solid. MS (ESI): mass calcd. for ci2Hi0N2O3, 230.07; m/z found, 231.0 [M+H]+
References:
WO2017/100668,2017,A1 Location in patent:Page/Page column 171