
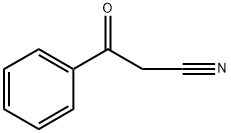
2-methyl-3-oxo-3-phenyl-propanenitrile synthesis
- Product Name:2-methyl-3-oxo-3-phenyl-propanenitrile
- CAS Number:7391-29-9
- Molecular formula:C10H9NO
- Molecular Weight:159.18
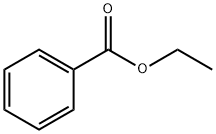
93-89-0
508 suppliers
$13.00/100g

107-12-0
4 suppliers
$24.69/1ML

7391-29-9
15 suppliers
$45.00/10mg
Yield:7391-29-9 98%
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran at 20; for 4 h;
Steps:
Intermediate 45 2-methyl-3-oxo-3-phenylpropanenitrile
A solution of propanenitrile (3.0 ml, 42 mmol) in THF (130 mL) was treated with lithium bis(trimethylsilyl)amide 1M in THF (120 mL, 1.0 M, 120 mmol). Subsequently ethyl benzoate (24 ml, 170 mmol) was added at room temperature. The mixture was stirred for 4 hours at room temperature. Water was added and the mixture was extracted with dichloromethane. The combined organic phases were discarded. The aqueous phase was acidified with aqueous hydrochloric acid and extracted with dichloromethane. The organic phase was washed with water, brine and dried over sodium sulfate. After removal of the solvent under reduced pressure 7.83 g (98% yield) of the desired product were obtained. LC-MS (method 10): Rt = 1.43 min; MS (ESIpos): m/z = 160 [M+H]+1H-NMR (400 MHz, DMSO-d6) δ [ppm]: 1.485 (15.70), 1.503 (16.00), 1.679 (2.50), 1.876 (11.52), 1.878 (13.95), 5.125 (1.69), 5.142 (5.29), 5.160 (5.26), 5.178 (1.64), 7.420 (0.40), 7.429 (0.41), 7.462 (0.76), 7.473 (3.07), 7.477 (3.42), 7.485 (6.48), 7.490 (7.04), 7.496 (1.65), 7.500 (1.30), 7.505 (2.29), 7.525 (1.49), 7.547 (1.82), 7.549 (2.38), 7.552 (2.14), 7.555 (2.00), 7.559 (2.21), 7.565 (1.64), 7.569 (1.70), 7.571 (1.78), 7.574 (1.47), 7.582 (3.45), 7.586 (1.47), 7.600 (7.32), 7.620 (5.31), 7.711 (2.34), 7.714 (1.39), 7.729 (3.48), 7.748 (1.35), 7.751 (0.75), 7.956 (0.68), 7.960 (1.26), 7.964 (1.23), 7.977 (0.93), 7.981 (1.24), 8.031 (6.27), 8.050 (6.00), 10.835 (3.58).
References:
WO2018/69222,2018,A1 Location in patent:Page/Page column 162

613-90-1
206 suppliers
$10.00/1g
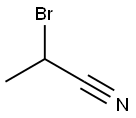
19481-82-4
77 suppliers
$24.00/1g

7391-29-9
15 suppliers
$45.00/10mg
614-16-4
255 suppliers
$17.00/5g

74-88-4
344 suppliers
$15.00/10g

7391-29-9
15 suppliers
$45.00/10mg
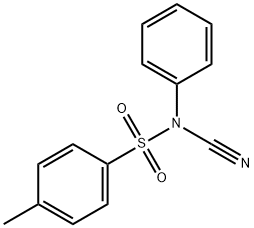
55305-43-6
65 suppliers
$23.00/100mg

93-55-0
7 suppliers
$15.00/25g

7391-29-9
15 suppliers
$45.00/10mg
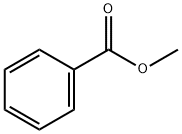
93-58-3
474 suppliers
$16.00/25mL

107-12-0
4 suppliers
$24.69/1ML

7391-29-9
15 suppliers
$45.00/10mg