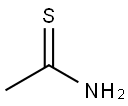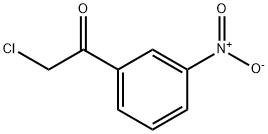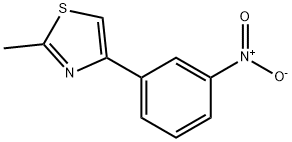
2-Methyl-4-(3-nitro-phenyl)-thiazole synthesis
- Product Name:2-Methyl-4-(3-nitro-phenyl)-thiazole
- CAS Number:39541-91-8
- Molecular formula:C10H8N2O2S
- Molecular Weight:220.25
Yield:39541-91-8 99%
Reaction Conditions:
in ethanol; for 2 h;Reflux;
Steps:
A Part A. 2-Methyl-4-(3-nitrophenyl)thiazole
2-bromo-l-(3-nitrophenyl)ethanone (2.00 g, 8.20 mmol), ethanethioamide (0.616 g, 8.20 mmol) and ethanol (50 mL) were combined and heated at reflux for 2 h. The reaction mixture was cooled to room temperature and concentrated. The product was tritrated with hexane containing a small amount of ethyl acetate. The suspension was cooled to 0 °C and the solid was collected on a Buchner funnel to give 2-methyl-4-(3-nitrophenyl)thiazole (1.78 g, 99% yield) as a colorless solid: 1H NMR (400 MHz, DMSO-d6) δ 8.73 (t, J=1.9 Hz, 1 H), 8.39 (d, J=7.8 Hz, 1 H), 8.27 (s, 1 H), 8.18 (dd, J=8.1, 2.3 Hz, 1 H), 7.73 (t, J=8.1 Hz, 1 H), 2.75 (s, 3 H); LCMS (ESI) m/e 221.2 [(M+H)+, calcd for C10H9N2O2S 221.0].
References:
WO2015/26574,2015,A1 Location in patent:Page/Page column 32-33

121-89-1
413 suppliers
$5.00/10g

39541-91-8
21 suppliers
$45.00/10mg