
2-METHYL-4-NITRO-1,3-BENZOXAZOLE synthesis
- Product Name:2-METHYL-4-NITRO-1,3-BENZOXAZOLE
- CAS Number:478553-83-2
- Molecular formula:C8H6N2O3
- Molecular Weight:178.14

603-85-0
266 suppliers
$15.00/5g
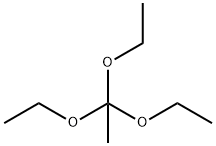
78-39-7
352 suppliers
$9.00/1g

478553-83-2
21 suppliers
$128.00/500mg
Yield:478553-83-2 95%
Steps:
474.A A.
A. 2-Methyl-4-nitrobenzoxazole (474A) To 2-amino-3-nitrophenol (6.17 g, 40.0 mmol) was added triethylorthoacetate (25.96 g, 160.0 mmol) and the mixture was heated at 100° C. for 12 h to give a dark red solution. Cooling to room temperature produced a crystalline mass which was filtered and washed with hexane to give compound 474A (6.78 g, 95%) as light maroon needles. HPLC: 98.1% at 1.86 min (retention time) (YMC S5 ODS column, 4.6*50 mm, eluding with 10-90% aqueous methanol over 4 min containing 0.2% phosphoric acid, 4 mL/min, monitoring at 220 nm). MS (ES): m/z 179.08 [M+H]+.
References:
US2004/77605,2004,A1 US2005/119228,2005,A1

603-85-0
266 suppliers
$15.00/5g
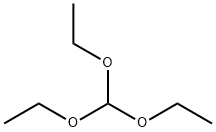
122-51-0
495 suppliers
$10.00/5ml

478553-83-2
21 suppliers
$128.00/500mg
![Acetamide, N-[2-(acetyloxy)-6-nitrophenyl]-](/CAS/20210305/GIF/69194-51-0.gif)
69194-51-0
0 suppliers
inquiry

478553-83-2
21 suppliers
$128.00/500mg
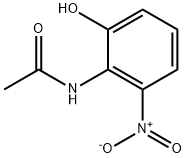
59820-29-0
1 suppliers
inquiry

478553-83-2
21 suppliers
$128.00/500mg

603-85-0
266 suppliers
$15.00/5g
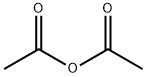
108-24-7
5 suppliers
$14.00/250ML

478553-83-2
21 suppliers
$128.00/500mg