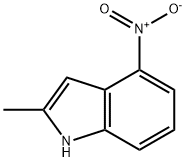
2-Methyl-4-nitroindole synthesis
- Product Name:2-Methyl-4-nitroindole
- CAS Number:3484-10-4
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
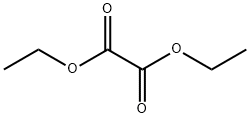
95-92-1
413 suppliers
$5.00/5 g

3484-10-4
93 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
Yield: 25% , 10%
Reaction Conditions:
with potassium ethoxide in dimethyl sulfoxide;N,N-dimethyl-formamide at 0; for 72 h;Reflux;
Steps:
2.2. 2-Methyl-4-nitroindole (410)
Without further purification, the imidate ester 3 was used in the reaction: in a 100 mL flask, 3.2 g (14.4 mmol) imidate ester in 12.0 mL anhydrous DMSO were treated with 3.0 mL diethyl oxalate (21.7 mmol, d = 1.076) in 8 mL anhydrous DMF and 1.58 g (18.8 mmol) potassium ethoxide at 0 °C. After 72 h at refluxing, the cooled red reaction mixture was poured onto ice/water and the nitro-indole separated as a red amorphous solid, which was collected and dried under vacuum at 30 °C. Subsequently it was purified by flash chromatography (ethyl acetate/n-hexane 1:1). Yield 25%; mp 192-94 °C; rf 0.63 (ethyl acetate/n-hexane 1:1); 1H NMR (DMSO-d6) δ 2.48 (s, 3H, CH3), 6.79 (d, 1H, J3,7 = 0.76, 3-H), 7.18 (dd, 1H, J6,7 = 8.01 Hz, J6,5 = 8.01 Hz, 6-H), 7.74 (dd, 1H, J7,6 = 8.01 Hz, J7,5 = 0.76 Hz, 7-H), 7.98 (dd, 1H, J5,6 = 8.01 Hz, J5,7 = 0.76 Hz, 5-H), 11.98 (br s, 1H, NH).
References:
Ferlin, Maria Grazia;Conconi, Maria Teresa;Urbani, Luca;Oselladore, Barbara;Guidolin, Diego;Di Liddo, Rosa;Parnigotto, Pier Paolo [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 1,p. 448 - 457] Location in patent:supporting information; experimental part
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

3484-10-4
93 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

3484-10-4
93 suppliers
inquiry

67-64-1
6 suppliers
$17.30/10ml
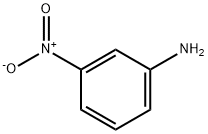
99-09-2
9 suppliers
$14.00/1g

3484-10-4
93 suppliers
inquiry

3484-23-9
71 suppliers
$12.00/100mg