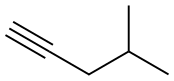
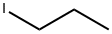2-Methyl-4-octyne synthesis
- Product Name:2-Methyl-4-octyne
- CAS Number:10306-94-2
- Molecular formula:C9H16
- Molecular Weight:124.22
Yield:10306-94-2 89%
Reaction Conditions:
Stage #1: 4-methylpentynewith n-butyllithium in tetrahydrofuran;hexanes at -78 - 20;
Stage #2: 1-iodo-propane in tetrahydrofuran;hexanes at 20; for 72 h;
Steps:
18.18-A
Examples 18 and 19; 1 -(4-(5 -(5 -Isobutyl-4-propylisoxazol-3 -yl)- 1 ,2,4-oxadiazol-3 -yl)benzyl)azetidine-3 - carboxylic acid, (18), and l-(4-(5-(4-Isobutyl-5-propylisoxazol-3-yl)-l,2,4-oxadiazol-3-yl)benzyl)azetidine-3- carboxylic acid, (19); 18-A. 2-Methyloct-4-yne; CH,H3C' ^ (18-A)[00226] To a solution of 4-methylpent-l-yne (0.588 mL, 5 mmol) in THF at -78 0C was added BuLi, 2.5 M in hexanes (2.200 mL, 5.50 mmol). After stirring 15 minutes at -78 0C, the reaction mixture was allowed to warm to room temperature, 1- iodopropane (0.488 mL, 5.00 mmol) was added and the reaction mixture was stirred at rt for 3 days. The reaction mixture was quenched with water (50 mL) and the resulting mixture was transferred to a separatory funnel. The mixture was extracted with ether (75 mL). The organic layer was washed with brine (50 mL), dried (MgSO4) and concentrated to afford 2-methyloct-4-yne (555 mg, 4.47 mmol, 89 % yield) as a light yellow liquid. 1H NMR (400 MHz, chloroform-d) δ ppm 0.91-0.99 (m, 9 H) 1.45-1.53 (m, 2 H) 1.70-1.81 (m, 1 H) 2.03 (dt, J=6.5, 2.4 Hz, 2 H) 2.12 (tt, J=I. Q, 2.4 Hz, 2 H).
References:
WO2010/85581,2010,A1 Location in patent:Page/Page column 110-111