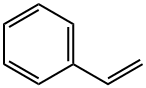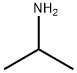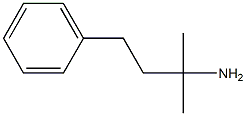
2-METHYL-4-PHENYLBUTAN-2-AMINE synthesis
- Product Name:2-METHYL-4-PHENYLBUTAN-2-AMINE
- CAS Number:43052-72-8
- Molecular formula:C11H17N
- Molecular Weight:163.2594
Yield:43052-72-8 93%
Reaction Conditions:
with eosin in acetonitrile at 20;Irradiation;Green chemistry;Reagent/catalyst;Solvent;Wavelength;
Steps:
General procedure for visible light mediated alkylation of amine substrates via direct HAT
General procedure: A flame-dried 10 mL flask was charged with amine 1 (1.0 mmol), substituted styrene 2 (1.0 mmol) and MeCN (3 mL). Eosin Y (2.0 mol%) was added to the mixture. The mixture was allowed to stir opening in air, and be irradiated with a high power blue LEDs [18 W, λ = 469 nm] for 8-12 hrs. at room temperature, until all of the starting material disappeared. The solvent was removed on a rotary evaporator under reduced pressure and the residue was subjected to column chromatography isolation on silica gel by elution with hexane to hexane / ethyl acetate (10:1) to afford the corresponding product 3(a-n) in high yield (65-97%) in Table 2. 2-Methyl-4-phenylbutan-2-amine (3a): m/z:163.14; 1H NMR (400 MHz, CDCl3) δ 7.25 - 7.23 (m, 2H), 7.18 - 7.14 (m, 3H), 2.64 - 2.61 (m, 2H), 1.68 - 1.65 (m, 2H), 1.15 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 142.8, 128.5, 128.4, 125.8, 49.8, 47.00, 31.2, 30.2. Anal. Calcd for C11H17N: C, 80.92: H, 10.50: N, 8.58. Found: C, 80.90: H, 10.48; N, 8.53.
References:
Srivastava, Vishal;Singh, Pravin K.;Singh, Praveen P. [Tetrahedron Letters,2019,vol. 60,# 19,p. 1333 - 1336] Location in patent:supporting information
79399-13-6
0 suppliers
inquiry

43052-72-8
5 suppliers
inquiry
![[(1E)-3-Azido-3-methyl-1-buten-1-yl]benzene](/CAS/20210305/GIF/1354701-45-3.gif)
1354701-45-3
0 suppliers
inquiry

43052-72-8
5 suppliers
inquiry
103-05-9
187 suppliers
$45.00/25g

43052-72-8
5 suppliers
inquiry