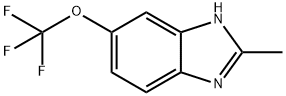
2-METHYL-5-TRIFLUOROMETHOXYBENZIMIDAZOLE synthesis
- Product Name:2-METHYL-5-TRIFLUOROMETHOXYBENZIMIDAZOLE
- CAS Number:114164-97-5
- Molecular formula:C9H7F3N2O
- Molecular Weight:216.16

658-89-9
78 suppliers
$50.00/1g

108-24-7
5 suppliers
$14.00/250ML

114164-97-5
17 suppliers
$55.00/250mg
Yield:114164-97-5 44%
Reaction Conditions:
Stage #1: 4-(trifluoromethoxy)benzene-1,2-diamine;acetic anhydride at 20;
Stage #2: with hydrogenchloride for 7 h;Reflux;
Stage #3: with ammonium hydroxide in water;
Steps:
2-Methyl-5-trifluoromethoxybenzimidazole. The starting material, 2-amino-4-trifluoromethoxyaniline, was obtained by the method of Yagupolskii, L. M. et al. (Yagupolskii L. M., Troitskaya V. I. J. Gen. Chem. USSR Engl. Transl., 1961, Vol. 31, p. 845; Chem. Abstr. 1961, Vol. 55, p. 22830f). The procedure of Philips, M. A. (Philips M. A. J. Chem. Soc. 1929, p. 2820-2828) was used to produce 2-methyl-5-trifluoromethoxybenzimidazole. To 2-amino-4-trifluoromethoxyaniline (0.7 g, 0.0036 Mol) acetic acid anhydride (1.5g, 0.0146 Mol) was carefully added at 20° C. and the mixture was stirred 5 minutes at this temperature. Then 2-3 drops of concentrated aqueous HCl was added with stiffing, and the mixture was refluxed for 7 hours The reaction solution was cooled and diluted with water (10 mL), 0.5 g of charcoal was added, and the mixture was refluxed more 5-10 min. After cooling, the mixture was filtered and resulted clear filtrate was washed with ether (2×10 mL). The water layer was neutralized with excess of dilute NH4OH -(charcoal was filtered) The precipitate was filtered, washed with water and dried to give a solid (0.34 g, 44% by wt. pure). M.p. 135-137° C. 1H NMR (DMSO-d6): 2.49 (s, 3H), 7.17 (d, J=8.5 Hz, 1H), 7.34 (s, 1H), 7.62 (d, J=8.5 Hz, 1H). 13C NMR (DMSO-d6): 14.47, 107.44, 114.19, 117.25, 120.28 (q, J=255.2 Hz), 137.06, 139.46, 143.09, 153.26. 19F NMR (DMSO-d6): -57.41. [M+1]+ 217.
References:
US8242284,2012,B1 Location in patent:Page/Page column 5-6