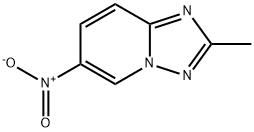
2-METHYL-6-NITRO(1,2,4)TRIAZOLO(1,5-A)PYRIDINE synthesis
- Product Name:2-METHYL-6-NITRO(1,2,4)TRIAZOLO(1,5-A)PYRIDINE
- CAS Number:7169-92-8
- Molecular formula:C7H6N4O2
- Molecular Weight:178.15
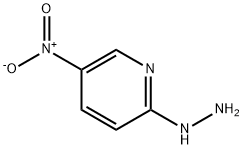
6343-98-2
76 suppliers
$35.00/250mg

64-19-7
1628 suppliers
$10.00/25ML

7169-92-8
11 suppliers
inquiry
Yield:7169-92-8 88%
Reaction Conditions:
at 110; for 48 h;
Steps:
General procedure for the preparation of compounds 15a-e and 16a-e.
General procedure: A 0.5M solution of 2-chloro-3(5)-nitropyridine (10 mmol) cooled on an ice bath was treated with hydrazine hydrate (50 mmol, added drop-wise over 1 h) and the resulting solution was stirred at room temperature overnight. The precipitate of 2-hydrazino-3(5)-nitropyridine was filtered off, washed with water and air-dried. It was dissolved in the respective aliphatic carboxylic acid and the solution (0.5 M) was heated at reflux for 48 h. Upon cooling to room temperature, the volatiles were removed in vacuo and the residue was triturated with 1M aqueous sodium bicarbonate. The precipitate thus formed was filtered off, washed with water and air dried. The resulting 1,2,4-triazolo[4,3-a]pyridine 14a-b was dissolved in 7M methanolic ammonia (0.25M with respect to 14a-b) and was hydrogenated over 10% Pd/C catalyst (0.1 equiv.) at 100 atm and 100 °C over 48 hours. Once it reached room temperature, the mixture was filtered through a short plug of silica gel and concentrated in vacuo. Chromatography on silica gel using 05% MeOH in CH2Cl2 afforded analytically pure compounds 15a-e or 16a-e.
References:
Mishchuk, Alexander;Shtil, Natalia;Poberezhnyk, Mykola;Nazarenko, Konstiantyn;Savchenko, Timur;Tolmachev, Andrey;Krasavin, Mikhail [Tetrahedron Letters,2016,vol. 57,# 9,p. 1056 - 1059] Location in patent:supporting information
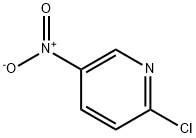
4548-45-2
546 suppliers
$6.00/5g

7169-92-8
11 suppliers
inquiry