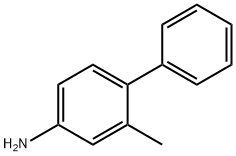
2'-METHYL-BIPHENYL-4-YLAMINE synthesis
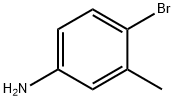
- Product Name:2'-METHYL-BIPHENYL-4-YLAMINE
- CAS Number:63019-97-6
- Molecular formula:C13H13N
- Molecular Weight:183.25
Yield:63019-97-6 100 %Chromat.
Reaction Conditions:
with potassium carbonate;palladium (II) chloride in lithium hydroxide monohydrate at 100; for 1.5 h;
Steps:
1 EXAMPLE 1
The following were charged into a 500 ml reactor provided with a coolant, a magnetic stirrer, a thermocouple and a nitrogen atmosphere: [00151] 28.8 g (150 mmole) of 97% 4-bromo-3-methylaniline; [00152] 21.1 g (153 mmole) of 100% potassium carbonate; [00153] 19.2 g (154 mmole) of 97% phenylboronic acid; [00154] 10 mg (0.056 mmole) of palladium chloride; [00155] 200 g (11.1 mole) of water, degassed conventionally by bubbling with nitrogen. [00156] The reactants were charged into the reactor and heated under reflux (100° C.) with stirring and under nitrogen. [00157] The reaction was complete after 1 h 30. [00158] Extraction was carried out with isopropyl ether (100 ml). [00159] It was quickly decanted. [00160] The organic phase was washed with 2×30 ml of water. [00161] It was dried over magnesium sulphate then concentrated by evaporation. [00162] High performance liquid chromatography analysis measured 28 g of 4-phenyl-3-methylaniline, corresponding to a 100% yield.
References:
US6800784,2004,B1 Location in patent:Page column 9
![[1,1'-Biphenyl]-2-carboxaldehyde, 4-nitro-](/CAS/20210305/GIF/124391-57-7.gif)
124391-57-7
0 suppliers
inquiry

63019-97-6
18 suppliers
inquiry
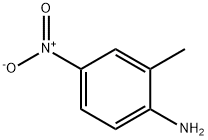
99-52-5
277 suppliers
$5.00/5G

63019-97-6
18 suppliers
inquiry