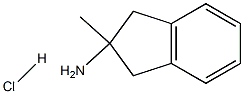
2-Methyl-indan-2-ylamine hydrochloride synthesis
- Product Name:2-Methyl-indan-2-ylamine hydrochloride
- CAS Number:91817-66-2
- Molecular formula:C10H14ClN
- Molecular Weight:183.6779

91142-58-4
14 suppliers
inquiry

91817-66-2
17 suppliers
inquiry
Yield:91817-66-2 51%
Reaction Conditions:
Stage #1: 2,3-dihydro-2-methyl-1H-indene-2-carboxylic acidwith sodium azide;chloroformic acid ethyl ester;triethylamine in water;acetone at 0; for 1.68333 h;
Stage #2: with hydrogenchloride in water;Reflux;
Steps:
A stirred solution of 2-methyl-2,3-dihydro-1 H-indene-2-carboxylic acid (1.63g, 9.26mmol, Description 5) in acetone (15ml) was cooled to O0C and treated with triethylamine (1.47ml, 10.46mmol) followed by the dropwise addition of ethyl chloroformate (1.00ml, 10.46mmol). The reaction mixture was stirred for 40 minutes at O0C. A solution of sodium azide (903mg, 13.89mmol) in water (5ml) was then added dropwise over 1 minute and stirring at O0C continued for a further 1 hour. The cooled suspension was then partitioned between ethyl acetate and saturated brine and the aqueous layer extracted with further ethyl acetate. The combined organic layers were dried over MgSO4 and evaporated to give an oil. A solution of this oil in toluene (10ml) was heated to 1000C for 20 minutes beyond the point at which nitrogen evolution ceased. The solvent was then removed and the residue heated to reflux in 5M hydrochloric acid (20ml) overnight. On cooling the reaction mixture was diluted with water and the insoluble material extracted with ethyl acetate. The organic layer was dried over MgSO4 and evaporated to afford recovered starting material (550mg, 34%). The aqueous reaction mixture was evaporated to dryness under reduced pressure to afford 2-methyl-2,3-dihydro-1 H-inden-2-amine hydrochloride as a white powder (870mg, 51%). 1H-NMR (400MHz, DMSO-d6): δ 8.42 (3H, broad s), 7.26 (2H, overlapping m), 7.19 (2H, overlapping m), 3.20 (2H, d, J = 16.4 Hz), 2.99 (2H, d, J = 16.4 Hz), 1.44 (3H, s).
References:
WO2010/57865,2010,A1 Location in patent:Page/Page column 25-26

934765-84-1
0 suppliers
inquiry

91817-66-2
17 suppliers
inquiry
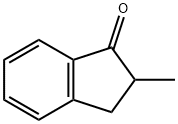
17496-14-9
90 suppliers
$20.00/250mg

91817-66-2
17 suppliers
inquiry

888326-91-8
0 suppliers
inquiry

91817-66-2
17 suppliers
inquiry

72181-95-4
6 suppliers
inquiry

91817-66-2
17 suppliers
inquiry