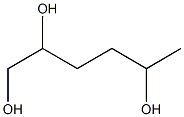
2-Methyl-tetrahydro-pyran-4-ol synthesis
- Product Name:2-Methyl-tetrahydro-pyran-4-ol
- CAS Number:89791-47-9
- Molecular formula:C6H12O2
- Molecular Weight:116.16

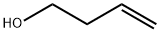
627-27-0
409 suppliers
$8.00/1g
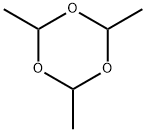
123-63-7
257 suppliers
$30.50/8182550100

89791-47-9
56 suppliers
$22.00/250mg
Yield: 7.5 g
Reaction Conditions:
with sulfuric acid at 85; for 48 h;
Steps:
17 Description 172-Methyltetrahydro-2H-pyran-4-ol (D17)
Description 172-Methyltetrahydro-2H-pyran-4-ol (D17)A mixture of but-3-en-1-ol (7.21 g), 2,4,6-trimethyl-1,3,5-trioxane (4.40 g) and 20% H2S04 (12 g)was heated to 85°C for 2 days. The mixture was cooled to RT and extracted with ether (4x50 mL).Combined organic layer was dried and evaporated to afford the title compound (7.5 g). MS (El):C6H,202 requires 116; found 116 [Mj.
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;GLAXOSMITHKLINE (CHINA) R&D COMPANY LIMITED;DENG, Jing;LEI, Hui;MA, Xin;LIN, Xichen WO2015/180612, 2015, A1 Location in patent:Page/Page column 47
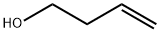
627-27-0
409 suppliers
$8.00/1g

75-07-0
424 suppliers
$14.00/5mL

89791-47-9
56 suppliers
$22.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

89791-47-9
56 suppliers
$22.00/250mg