
2-METHYLHEPTADECANE synthesis
- Product Name:2-METHYLHEPTADECANE
- CAS Number:1560-89-0
- Molecular formula:C18H38
- Molecular Weight:254.49
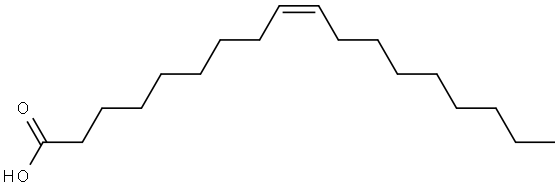
112-80-1
1044 suppliers
$5.00/10g
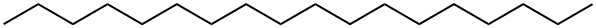
593-45-3
269 suppliers
$17.00/25g

1560-89-0
16 suppliers
$505.85/5MG
Yield:593-45-3 58 %Chromat. ,1560-89-0 28 %Chromat.
Reaction Conditions:
with hydrogen at 360; under 15001.5 Torr; for 1 h;Green chemistry;Reagent/catalyst;
Steps:
Catalytic activities of Pd/Z and FPd/Z on thehydrodeoxygenation and isomerization of oleic acid into biofuel
Recently in attempt to gain thorough insight into thehydrodeoxygenation (HDO) process of different feed stocks, stud-ies on reaction variables such as effects of reaction pressure andtemperature, H2gas flow rate, catalyst loading and even typesof reactors and mode of operations have been well studied andreported [20,30-34]. However, since existing hydrotreating unitsof conventional crude oil refineries can be adapted for the HDOprocess of biofuel production, studies on expedient synthesis ofsuitable catalysts such as types of the support and metals [34],systematic catalyst preparation procedure [20,26,35] and additivessuch as sulfur [36] and phosphorus [31] are currently receivingattention. In this direction, we recently, functionalized nickel withoxalic acid and supported on alumina for the hydrodeoxygenationof oleic acid into high grade biofuel [18]. At the best operationalconditions of 20 bar, 360C and 100 ml/min of gas flow, the catalystshowed superior activities to other alumina supported Ni catalystsin literature. In addition, the catalyst was able to achieve skeletalisomerization of already hydrodeoxygenated n-paraffin due to theeffect of the oxalic acid functionalization.As a step further, in this work we modified palladium oxalatecatalyst with fluoride ion and compared its activities with unmod-ified palladium oxalate catalyst at the earlier best observedoperation condition for the HDO of oleic acid and the result is shownin Fig. 8. It is very obvious that FPd/Z possesses both higher HDO andisomerization activities compared to Pd/Z. Even though Pd/Z andFPd/Z showed almost same n-C18H38quantity, but the fact that thelatter has superior isomerization ability confirmed that it is moreprospective for industrial application since it is well known thatn-C18H38produced in the HDO step is the substrate for the isomer-ization step to produce the iso-C18H38. The enhanced HDO activityof FPd/Z compared to Pd/Z was ascribed to its increased acidity atthe synthesis stages which improves the solubility of Pd in the sup-port and facilitates the formation of Pd (II) polynuclear complexwhich in turn guarantees high Pd dispersion as seen in the XRDresult (Fig. 4). In addition, its increased acidity also modified itsmorphology from crystalline into amorphous (Fig. 2 (insets)) andenhanced its textural properties (Table 2) which in turn increasesthe N2adsorption/desorption isotherm hysteresis loop as shownin Fig. 3. This observation is in agreement with the works of Liet al. [26] on the functionalization of NiMo/-Al2O3catalysts withvarying quantities of urea. They reported that the urea functional-ization greatly enhances the textural properties and the solubilityof Mo and Ni particles in the support as well as facilitates the for-mation of molybdate and polymolybdate species due to increasedacidity.Generally, aluminosilicates gradually transform from crystallineform into amorphous under acid attacks [19,37] thus leading toincrease in Si/Al ratio as seen in Table 1. Such transformationis usually accompanied with reduction in particle size which inturn increased the specific surface area and porosity as observedin Table 2. Such morphological and textural properties variationhas been reported to increase catalyst activities by ensuring welldispersed active metal particles [22,26]. Therefore, the enhancedtextural properties of FPd/Z compared to Pd/Z will equally provideadditional and sufficient surface area for active metal incorporationand also guarantee unrestricted access for the reacting moleculesowing to enhanced porosity [19,22]. Furthermore, both catalystswere seen to produce isomerized products, while FPd/Z producesabout 28% iso-C18H38, Pd/Z produces only 11%. The presence ofthe iso-C18H38was due to FPd/Z increased acidity. Previous stud-ies [32,35] have shown that catalysts with increased acidity arehighly favorable for paraffin skeletal isomerization. Typically, thepresence of isomerized product is considered an advantage dueto the ability of iso-paraffin fractions to lower the biofuel freez-ing point by about 20C since n-paraffin (C16-C18) has comparablehigh freezing point (between 18C and 28C) which is a disad-vantage to their cold flow properties such as cold filter pluggingpoint [32,38]. The increased iso-C18H38content in FPd/Z comparedto Pd/Z was unarguably due to the fluoride ion functionalizationwhich further increased the acidity of FPd/Z. The presence of C17H36in Fig. 8 when using Pd/Z confirmed instances of decarboxyla-tion or decarbonylation, and this is further corroborated by theincrease in the amount of gases (such as CO2/CO). Unfortunately,as earlier commented (Section 2.4) the online identification andquantification of these gases were not technically feasible dur-ing this study, hence determination of the gas species was notpossible. The amount of the lumped unidentified products suchas unreacted oleic acid, oligomerized products and other func-tional group is also higher in Pd/Z showing that its activity andselectivity is comparably inferior to FPd/Z. It is obvious that effectof fluoride ion functionalization is highly invaluable since onlythe iso-paraffin content of FPd/Z fall within the range (20-40%)reported bySimcek et al. [39] that could significantly improvethe cold flow properties (CFPP, pour point, cloud point, etc.) ofbiofuel.
References:
Ayodele;Farouk, Hamisu U.;Mohammed, Jibril;Uemura, Yoshimitsu;Daud [Journal of Molecular Catalysis A: Chemical,2015,vol. 400,p. 179 - 186]

3761-92-0
110 suppliers
$55.00/100ml

124388-95-0
0 suppliers
inquiry

1560-89-0
16 suppliers
$505.85/5MG
20194-45-0
12 suppliers
$185.00/250mg

1560-89-0
16 suppliers
$505.85/5MG
35354-37-1
87 suppliers
$18.00/100mg

1560-89-0
16 suppliers
$505.85/5MG

2243-98-3
72 suppliers
$98.00/5mL

1560-89-0
16 suppliers
$505.85/5MG