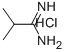
2-METHYLPROPANIMIDAMIDE HYDROCHLORIDE synthesis
- Product Name:2-METHYLPROPANIMIDAMIDE HYDROCHLORIDE
- CAS Number:22007-68-7
- Molecular formula:C4H11ClN2
- Molecular Weight:122.6

52070-18-5
16 suppliers
$266.00/250mg

22007-68-7
106 suppliers
$12.00/250mg
Yield:-
Reaction Conditions:
with ammonia in ethanol; for 5 h;Green chemistry;
Steps:
General Procedure of the One-Pot Syntheses of2-Substituted 6-Hydroxy-[3H]-pyrimidin-4-ones 4
General procedure: Dry hydrogen chloride (1.3 mol) was added to an ice-cooled solution of 1 mol nitrile and 1.3 mol ethanol. The solution was stirred for two days at room temperature. A solution of 25 g (1.5 mol) of ammonia in absolute ethanol was prepared and added to the formed imido acid ethyl ester solution. The reaction mixture was stirred for 5 h, the precipitated NH4Cl was filtered off, and the clear solution was stored in a closed flask. A sodium ethanolate solution was prepared with 46 g (2 mol) of sodium in1 L of ethanol. The amidine hydrochloride-ethanol solution and 160 g (1 mol) of diethyl malonate were added. A white precipitate was immediately formed and the mixture was refluxed for 4 h. The solvent was distilled off under reduced pressure, and the crude sodium salt was dissolved in 1 L of water. From the clear solution the 2-substituted 6-hydroxy-[3H]-pyrimidin-4-one was precipitated by addition of aqueous concentrated hydrochloric acid. The precipitate was isolated, washed with distilled water, and dried under vacuum at 80°C for 6 h.
References:
Opitz, Andreas;Sulger, Werner;Daltrozzo, Ewald;Koch, Rainer [Australian Journal of Chemistry,2015,vol. 68,# 5,p. 814 - 824]
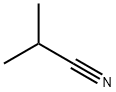
78-82-0
8 suppliers
$14.14/5gm:

22007-68-7
106 suppliers
$12.00/250mg