2-Morpholino-5-nitrobenzotrifluoride synthesis
- Product Name:2-Morpholino-5-nitrobenzotrifluoride
- CAS Number:54672-11-6
- Molecular formula:C11H11F3N2O3
- Molecular Weight:276.21

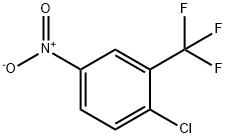
110-91-8
678 suppliers
$9.00/1g

400-74-8
213 suppliers
$14.00/25g

54672-11-6
15 suppliers
$100.00/1g
Yield:54672-11-6 94.2%
Reaction Conditions:
in dimethyl sulfoxide at 100; for 5 h;
Steps:
4.2.11.1. 4-(4-nitro-2-(trifluoromethyl) phenyl) morpholine (4a)
1-fluoro-4-nitro-2-(trifluoromethyl)benzene (2.1 g, 10 mM) and morphine (2.6 mL, 30 mM) in DMSO (20 mL) were heated to 100°C for 5 h. The mixture was partitioned between ethyl acetate (80×2 mL) and water (40×2 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and evaporated to get 4a (2.6 g, 9.4 mM) as yellow solid in 94.2% yields. ESI-MS m/z: 277.1 [M+H] +.
References:
Wang, Lu;Zhang, Qing;Zhu, Gaoyuan;Zhang, Zhimin;Zhi, Yanle;Zhang, Li;Mao, Tianxiao;Zhou, Xiang;Chen, Yadong;Lu, Tao;Tang, Weifang [European Journal of Medicinal Chemistry,2017,vol. 130,p. 86 - 106] Location in patent:supporting information