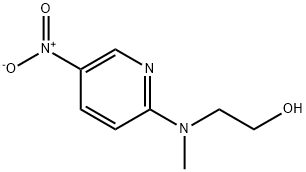
2-[N-methyl-N-(5-nitro-2-pyridyl)amino]ethanol synthesis
- Product Name:2-[N-methyl-N-(5-nitro-2-pyridyl)amino]ethanol
- CAS Number:25948-15-6
- Molecular formula:C8H11N3O3
- Molecular Weight:197.19

109-83-1
432 suppliers
$10.00/25ml
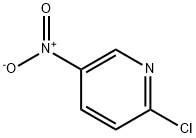
4548-45-2
534 suppliers
$6.00/5g
![2-[N-methyl-N-(5-nitro-2-pyridyl)amino]ethanol](/CAS/GIF/25948-15-6.gif)
25948-15-6
6 suppliers
$28.60/1g
Yield:25948-15-6 91%
Reaction Conditions:
at 20; for 1 h;
Steps:
90.1 Step 1:
2-chloro-5-nitropyridine (4.0 g) was stirred with 2-methylaminoethanol (20 mL) at room temperature for 1 h. The reaction mixture was diluted with water (30 mL) and extracted with ethyl acetate (50 mL x 2), washed with brine (20 mL), dried over Na2S04 and evaporated under vacuum. The residue was washed with n-pentane (25 mL) to get 2-(methyl(5- nitropyridin-2-yl)amino)ethanol (4.5 g, 91 %, yellow solid). TLC system: methanol/chloroform (1 :19), Rf: 0.4.
References:
WO2013/13817,2013,A1 Location in patent:Page/Page column 121
5418-51-9
440 suppliers
$12.72/1gm:
![2-[N-methyl-N-(5-nitro-2-pyridyl)amino]ethanol](/CAS/GIF/25948-15-6.gif)
25948-15-6
6 suppliers
$28.60/1g