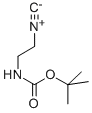
2-(N-T-BUTOXYCARBONYLAMINO)ETHYLISOCYANIDE synthesis
- Product Name:2-(N-T-BUTOXYCARBONYLAMINO)ETHYLISOCYANIDE
- CAS Number:215254-91-4
- Molecular formula:C8H14N2O2
- Molecular Weight:170.21
![Carbamic acid, [2-(formylamino)ethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/215254-90-3.gif)
215254-90-3
11 suppliers
inquiry

215254-91-4
20 suppliers
inquiry
Yield:215254-91-4 89%
Reaction Conditions:
with iodine;N-ethyl-N,N-diisopropylamine;triphenylphosphine in dichloromethane at 20; for 1 h;
Steps:
Isocyanides 2; General Procedure
General procedure: To a stirred solution of formamide 1 (1.0 mmol) and I2 (381 mg, 1.5mmol) in CH2Cl2 (3 mL) was added Ph3P (394 mg, 1.5 mmol), followedby the dropwise addition of Et3N (415 μL, 301 mg, 3 mmol). The mixturewas stirred at r.t. until the consumption of the formamide wascomplete (TLC monitoring, typically 1 h), and then diluted withCH2Cl2 (10 mL) and washed with ice-cold sat. aq Na2S2O3 solution (10mL). The aq phase was extracted with CH2Cl2 (2 × 10 mL). Each portionof the organic phase was sequentially washed with deionizedH2O (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and filtered.The combined organic phase was concentrated under reducedpressure and purified by column chromatography (silica gel, typicallyPE as the eluent) to give the corresponding isocyanide 2. Any deviationsfrom this procedure are noted in Table 2 and Scheme 1.
References:
Wang, Xia;Wang, Qing-Gang;Luo, Qun-Li [Synthesis,2015,vol. 47,# 1,p. 49 - 54]
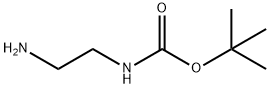
57260-73-8
469 suppliers
$5.00/5g

215254-91-4
20 suppliers
inquiry

24424-99-5
823 suppliers
$13.50/25G

215254-91-4
20 suppliers
inquiry