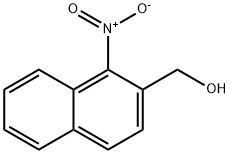
2-Naphthalenemethanol, 1-nitro- synthesis
- Product Name:2-Naphthalenemethanol, 1-nitro-
- CAS Number:265126-05-4
- Molecular formula:C11H9NO3
- Molecular Weight:203.19
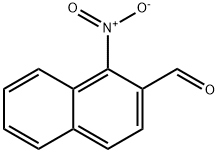
101327-84-8
33 suppliers
$60.00/5mg

265126-05-4
3 suppliers
inquiry
Yield:265126-05-4 99%
Reaction Conditions:
with sodium tetrahydridoborate in ethanol at 0 - 20; for 2 h;
Steps:
Using the general procedure for the reduction of substituted 2-nitrobenzaldehyde the desired product was obtained as a dark yellow solid, 1 g, >99 % yield. R.f. 0.59 (EtOAc), m.p 78-80 °C,1H NMR (CDCl3, 270 MHz,): δ 2.32 (IH, br.s, OH), 4.82 (2H, s, CH2), 7.48-7.65 (3H, m, ArH), 7.81-7.90 (2H, m, ArH), 7.99 (IH, d, J= 8.4, ArH). General Procedure for the Reduction of Substituted 2-Nitrobenzaldehyde.solution of the desired substituted 2-nitrobenzaldehyde in EtOH (5 ml/mrnol) was cooled to 00C and to this was added NaBH4 (1.5 eq) and the resulting solution was stirred at r.t. for 2 h. The EtOH was removed in vacuo and sat. NH4Cl solution was added and
References:
WO2009/66072,2009,A2 Location in patent:Page/Page column 55-56; 66