2-nitro-4-propoxyaniline synthesis
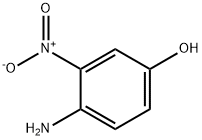
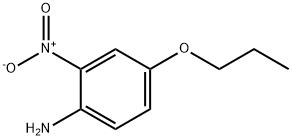
- Product Name:2-nitro-4-propoxyaniline
- CAS Number:20367-34-4
- Molecular formula:C9H12N2O3
- Molecular Weight:196.2
Yield:20367-34-4 80%
Reaction Conditions:
with lithium hydroxide monohydrate in ethanol at 70;
Steps:
2-Nitro-4-propoxyaniline (35a).
2-Nitro-4-propoxyaniline (35a). A 250 mL 3-neck flask fitted with a stir-bar and an Ar inlet was charged with 4-amino-3-nitrophenol (5.00 g, 32.4 mmol), n-propyl bromide (5.59 g, 4.13 mL, 45.4 mmol), LiOH H2O (2.72 g, 64.8 mmol), and EtOH (50 mL). The mixture was heated at 70 °C overnight. Another 0.5 mL n-propyl bromide was added and the mixture was further heated for 5 hr. An additional 500 mg LiOH H2O and 0.5 mL n-propyl bromide were added and the mixture was heated overnight. After cooling, the solution was partitioned with H2O (100 mL) and EtOAc (150 mL). The organic phase was washed with 1 % aqueous LiOH (2 χ 100 mL), H2O (100 mL), and brine (100 mL), filtered through phase separation paper, and concentrated in vacuo to give 6.3 g of a red solid. The crude material was triturated with hexanes:EtOAc 10:1 (30 mL), filtered, and pressed with rubber dam. The solid was rinsed with hexanes (2 x 10 mL) to give 5.1 g 35a as a red solid (80%). HPLC analysis (15:10:75 H2O:A1 :MeOH) showed a purity of 97% with a retention time of 5.3 min.
References:
WO2013/101926,2013,A1 Location in patent:Page/Page column 32-33

20367-33-3
5 suppliers
inquiry

20367-34-4
11 suppliers
inquiry

20367-32-2
13 suppliers
inquiry

20367-34-4
11 suppliers
inquiry