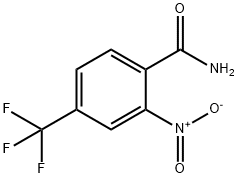
2-NITRO-4-TRIFLUOROMETHYLBENZAMIDE 97 synthesis
- Product Name:2-NITRO-4-TRIFLUOROMETHYLBENZAMIDE 97
- CAS Number:22227-55-0
- Molecular formula:C8H5F3N2O3
- Molecular Weight:234.13
778-94-9
188 suppliers
$10.00/5g

22227-55-0
23 suppliers
$100.00/5g
Yield:22227-55-0 99%
Reaction Conditions:
with sulfuric acid;water for 3 h;Heating / reflux;
Steps:
14-1
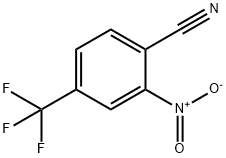
Example 14-1: Preparation of 2-Nitro-4-trifluoromethyl-thiobenzamide (55).; 2-Nitro-4-trifluoromethyl-benzonitrile (3.9 g, 18 mmol) was dissolved in 70% aqueous H2SO4 and the reaction heated to reflux for 3 h. The reaction mixture was slowly poured on ice (300 ml) with vigorous stirring. The solution was filtered and the solid washed with cold water and hexane. The solid material was subsequently redissolved in ethyl acetate and the solution washed with 10% aqueous NaHCO3 solution, brine, dried (MgSO4) and filtered. Removal of the solvent in vacuo afforded 2-nitro-4- trifluorobenzamide (4.22 g, 99%). LC/MS (Method F): tR= 1.66 min, >95%, m/z (ESI+)= 218(M-NH2)+. The afforded benzamide (4.22 g, 18 mmol) was dissolved in dioxane (200 ml) and phosphorous pentasulfide (3.4 g, 15 mmol) was added. The reaction was heated to 110°C for 4 hours after which time no starting material could be detected. The solvent was removed in vacuo and the residue partitioned between DCM and 10% aqueous NaHCO3. The phases were separated and the organic phase was washed with brine, dried (MgSO4), filtered and concentrated to afford an oil, which was further purified by flash chromatography (Silica, ethyl acetate/hexane) to afford the target thio-benzamide, (3.63 g, 81%). LC/MS: (Method F): tR= 2.21 min, >95%, m/z (ESI+)= 234 (M-NH2)+.
References:
WO2007/14922,2007,A1 Location in patent:Page/Page column 81