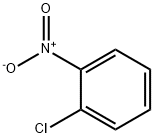
2-Nitrochlorobenzene synthesis
- Product Name:2-Nitrochlorobenzene
- CAS Number:88-73-3
- Molecular formula:C6H4ClNO2
- Molecular Weight:157.55
88-72-2
248 suppliers
$15.00/25g

88-73-3
8 suppliers
$16.00/25g
Yield:88-73-3 86.1%
Reaction Conditions:
with bromine;iodine;chlorine at 140; for 2 h;Reagent/catalyst;Temperature;
Steps:
32 Example 32
In a 1000 ml four-necked round bottom flask, 312.56 g o-nitrotoluene and a quantitative catalyst (a mixture of 10: 1 mass ratio of bromine and elemental iodine) were added. The mass ratio of o-nitrotoluene to the catalyst was controlled at1: 0.08,235r / min speed stirring and heated to 140 ° C, slowly into the chlorine gas, two hours after the sample, with gas chromatography tracking reaction by-products o-nitrobenzylidene chloride to 5%O-nitrotoluene conversion of 41%, the reaction was stopped; After completion of the reaction, the degree of vacuum -0.098MPa, vacuum distillation at a temperature of 109 deg.] C, recovering nitrotoluene, continued use of chlorinated cyclic;The residue after recovery of o-nitrotoluene was recrystallized from petroleum ether to obtain o-nitrochlorobenzyl chloride as a white crystalline solid.
In this example, the production effect of 99.2% of o-nitrochlorobenzyl chloride and 86.1% of yield (based on the actual consumed o-nitrotoluene) was achieved.
References:
Sichuan Fusida Biotechnology Development Co., Ltd.;Liu, Hu;Wang, Wen;Ma, Qingwei;Chen, Xi;Li, Zhou;Zhang, Hua;Wang, Lei CN106008348, 2016, A Location in patent:Paragraph 0119; 0120
95-51-2
371 suppliers
$5.00/5 g

88-73-3
8 suppliers
$16.00/25g

577-19-5
9 suppliers
$14.00/5g

88-73-3
8 suppliers
$16.00/25g
552-16-9
337 suppliers
$10.00/10g

88-73-3
8 suppliers
$16.00/25g

3900-89-8
377 suppliers
$6.00/1g

88-73-3
8 suppliers
$16.00/25g