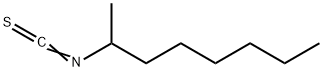
2-OCTYL ISOTHIOCYANATE synthesis
- Product Name:2-OCTYL ISOTHIOCYANATE
- CAS Number:69626-80-8
- Molecular formula:C9H17NS
- Molecular Weight:171.3

75-15-0
235 suppliers
$40.00/100g
693-16-3
110 suppliers
$19.00/250mg

69626-80-8
27 suppliers
$39.00/1g
Yield:69626-80-8 97%
Reaction Conditions:
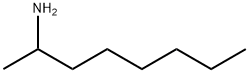
Stage #1: carbon disulfide;rac-2-aminooctanewith triethylamine in dichloromethane at 20; for 0.0833333 h;
Stage #2: with 4‐(4,6‐dimethoxy‐1,3,5‐triazin‐2‐yl)‐4‐methylmorpholinium toluene‐4‐sulfonate in dichloromethane at 90; for 0.05 h;Microwave irradiation;
Steps:
3.2.1. General Procedure for Compounds 4a-j-Method A
General procedure: Amine 2a-j (2 mmol, 1 equiv.), Et3N (0.84 mL, 6 mmol, 3 equiv. or 1.68 mL, 12 mmol,6 equiv. for 2j), and CS2 (0.36 mL, 6 mmol, 3 equiv. or 0.72 mL, 12 mmol, 6 equiv. for2j) were dissolved in dry DCM (3 mL or 5 mL for 2j) in a 10 mL pressure vial, equippedwith a magnetic bar, and stirred 5 min at rt. Next, DMT/NMM/TsO- (1) (0.828 g, 2 mmol,1 equiv. or 1.656 g, 4 mmol, 2 equiv. for 2j) was added. The reaction was carried under MWconditions (standard mode, 3 min, 90 °C). The reaction mixture was diluted with DCM(50 mL) and washed with H2O (5 mL), 1 N HCl (2 x 5 mL), H2O (5 mL), then dried underanhydrous MgSO4. The crude products were purified by flash chromatography on silicagel (7-8 g) using hexane as an eluent. Pure isothiocyanates 4a-j were isolated after carefulevaporation of the solvent and removal of volatile residues under reduced pressure. Allthe synthesized isothiocyanates have been described in the literature.
References:
Janczewski, ?ukasz;Kolesińska, Beata;Kr?giel, Dorota [Molecules,2021,vol. 26,# 9]

693-16-3
110 suppliers
$19.00/250mg

69626-80-8
27 suppliers
$39.00/1g