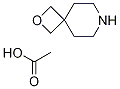
2-oxa-7-azaspiro[3.5]nonane acetate synthesis
- Product Name:2-oxa-7-azaspiro[3.5]nonane acetate
- CAS Number:1313369-52-6
- Molecular formula:C9H17NO3
- Molecular Weight:187.2362
![2-Oxa-7-azaspiro[3.5]nonane](/CAS/GIF/241820-91-7.gif)
241820-91-7
124 suppliers
$125.00/50mg

64-19-7
1535 suppliers
$10.00/25ML
![2-oxa-7-azaspiro[3.5]nonane acetate](/CAS/GIF/1313369-52-6.gif)
1313369-52-6
13 suppliers
inquiry
Yield:1313369-52-6 8.9 g
Reaction Conditions:
in dichloromethane at 0; for 0.25 h;
Steps:
2.4. Preparation of compound 1a (HOAc salt)
To a solution of compound 15 (15.0 g, 57.4 mmol) in MeOH (150 mL) was added wet Pd/C (10%/w, 1.5 g). The reaction was stirred under H2 balloon at rt overnight. The reaction mixture was filtered through a pad of celite and the filtrate was concentrated to give the crude product (7.0 g), which was dissolved in DCM (200 mL). HOAc (3.5 g, 57.9 mmol) was added and the mixture was stirred at 0 °C for 15 min and concentrated at 0 °C to get a solid. The solid was further washed with PE: EtOAc = 20:1 (50 mL) to afford 1a (8.9 g, 83%) as HOAc salt (a white solid).1H NMR (DMSO-d6): δ 1.70 (t, 4H, J = 5.6 Hz) 1.75 (s, 3H) 2.64 (t, 4H, J = 5.6 Hz) 4.20 (s, 4H).13C NMR (DMSO-d6): δ 23.3 34.7 38.9 42.6 81.5 173.9.HRMS for C7H13NO+ [M+H]+ Calcd 128.1070; Found 128.1068.
References:
Xu, Ruo;Czarniecki, Michael;Man, Jos De;Pan, Jianping;Qiang, Li;Root, Yuriko;Ying, Shihong;Su, Jing;Sun, Xijun;Zhang, Yuping;Yu, Tao;Zhang, Yang;Hu, Tao;Chen, Shu-Hui [Tetrahedron Letters,2011,vol. 52,# 26,p. 3266 - 3270] Location in patent:experimental part
![benzyl 2-oxa-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/20200401/GIF/1313369-55-9.gif)
1313369-55-9
1 suppliers
inquiry
![2-oxa-7-azaspiro[3.5]nonane acetate](/CAS/GIF/1313369-52-6.gif)
1313369-52-6
13 suppliers
inquiry
848070-26-8
12 suppliers
inquiry
![2-oxa-7-azaspiro[3.5]nonane acetate](/CAS/GIF/1313369-52-6.gif)
1313369-52-6
13 suppliers
inquiry
374794-84-0
35 suppliers
inquiry
![2-oxa-7-azaspiro[3.5]nonane acetate](/CAS/GIF/1313369-52-6.gif)
1313369-52-6
13 suppliers
inquiry
![2-Oxa-7-azaspiro[3.5]nonane-7-carboxylic acid, 1,1-dimethylethyl ester](/CAS/GIF/240401-27-8.gif)
240401-27-8
83 suppliers
$107.00/100mg
![2-oxa-7-azaspiro[3.5]nonane acetate](/CAS/GIF/1313369-52-6.gif)
1313369-52-6
13 suppliers
inquiry