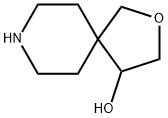
2-Oxa-8-azaspiro[4.5]decan-4-ol (9CI) synthesis
- Product Name:2-Oxa-8-azaspiro[4.5]decan-4-ol (9CI)
- CAS Number:777049-50-0
- Molecular formula:C8H15NO2
- Molecular Weight:157.21
![2-Oxa-8-azaspiro[4.5]decan-4-ol (9CI)](/CAS/GIF/777049-50-0.gif)
777049-50-0
24 suppliers
$90.00/10mg

24424-99-5
822 suppliers
$13.50/25G
![TERT-BUTYL 4-HYDROXY-2-OXA-8-AZASPIRO[4.5]DECANE-8-CARBOXYLATE](/CAS/20180703/GIF/CB84031158.gif)
757239-42-2
4 suppliers
inquiry
Yield:757239-42-2 90%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 2 h;
Steps:
tert-Butyl 4-hydroxy-2-oxa-8-azaspiro[4.5]decane-8-carboxylate
tert-Butyl 4-hydroxy-2-oxa-8-azaspiro[4.5]decane-8-carboxylate (0326) To a solution of 2-oxa-8-azaspiro[4.5]decan-4-ol (1.0 g, 6.4 mmol) in CH2Cl2 (15 mL), was added di-tert-butyl dicarbonate (1.7 g, 7.6 mmol) at RT, then Et3N (1.2 mL, 12.8 mmol) was added at RT. The reaction mixture was stirred at RT for 2 h, quenched with H2O (5 mL) and extracted with EtOAc (15 mL×3). The organic layer was separated and washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure to give the title compound as a colorless oil (1.5 g, 90%), which was used directly without further purification. (0327) MS (ES+) C13H23NO4 requires: 257, found: 280 [M+Na]+.
References:
US2017/342078,2017,A1 Location in patent:Paragraph 0326; 0327