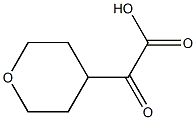
2-oxo-2-(tetrahydro-2H-pyran-4-yl)acetic acid synthesis
- Product Name:2-oxo-2-(tetrahydro-2H-pyran-4-yl)acetic acid
- CAS Number:871261-02-8
- Molecular formula:C7H10O4
- Molecular Weight:158.1519
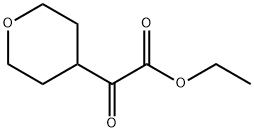
861160-58-9
6 suppliers
inquiry

871261-02-8
8 suppliers
inquiry
Yield:871261-02-8 95.3%
Reaction Conditions:
with potassium hydroxide;dipotassium hydrogenphosphate;water at 20; for 2 h;
Steps:
3
Example 3 From compound 17b (THP a -ketoester) to compound 18 (THP α-Ketoacid); The organic solution containing THP a -ketoester 17b (1.07 kg, 5.79 mol) was added to a solution containing water (3.5 L), KOH (89%, 486 g, 7.70 mol) and K2HP04 (435 g, 2.50 mol). Use of a buffered hydrolysis system minimizes side-products. This was stirred at RT for 2 h, then the organic layer was cut. The aqueous layer was then concentrated to remove EtOH. To the resulting solution was added a 3:1 MTBE/ THF mixture (5.0 L) and this solution was cooled to 10 °C. Concentrated HCl (1.0 L) was added, followed by NaCl (500 g). The organic layer was collected, then the aqueous layer was washed with 3:1 MTBE/ THF (2 x 5.0 L). The resulting combined organics was stripped to a thick residue, then was flushed with MTBE (4.0 L). The resulting solution was filtered through an in-line filter, then concentrated to 1.7 L and heated to 49 °C to dissolve all solids. The solution was cooled to 45 °C, then seed was added. Upon cooling to 40 °C, a slurry formed. n-Heptane (5.1 L) was then added over 2 h at 40 °C. The mixture was then cooled to 5 °C over 50 min and filtered rinsing the solids with n- heptane (1.0 L). After drying at 50 °C in a vacuum oven, 891.4 g (95.3% yield from 17b) of off-white solid was obtained (99.3 A%, 97.9 wt%). The overall yield of 18 from 16 was 71.7%. HPLC Assay Column: Zorbax RX-C8, 4.6 mm X 250 mm; Solvents: 50% CH3CN, 50% 0.25% HC104; Flow: 1.0 mL/min; Temperature: 25 °C; Sample volume: 5 μ L; Wavelength: 210 nm (Ref 360,100); Retention times: a -Ketoacid 18: 2.7 min; α-Ketoester 17b: (broad) 4.7min.
References:
WO2005/118529,2005,A2 Location in patent:Page/Page column 19
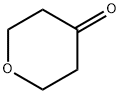
29943-42-8
424 suppliers
$5.00/1g

871261-02-8
8 suppliers
inquiry
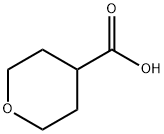
5337-03-1
301 suppliers
$10.00/1g

871261-02-8
8 suppliers
inquiry