
2-Oxo-4-phenylbutyric acid methyl ester synthesis
- Product Name:2-Oxo-4-phenylbutyric acid methyl ester
- CAS Number:83402-87-3
- Molecular formula:C11H12O3
- Molecular Weight:192.21

67-56-1
771 suppliers
$9.00/25ml
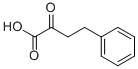
710-11-2
192 suppliers
$20.00/500mg

83402-87-3
15 suppliers
$80.00/100mg
Yield:83402-87-3 100%
Reaction Conditions:
with hydrogenchloride for 3.5 h;Reflux;
Steps:
8
Acetyl chloride (0.4 mL, 5.61 mmol) was slowly added dropwise to an anhydrous methanol solution (8 mL) in an ice bath.The mixture was stirred at room temperature for 1 hour to prepare an anhydrous methanolic hydrogen chloride solution.The freshly prepared methanol solution of anhydrous hydrogen chloride was slowly added dropwise to a solution of 2-oxo-4-phenylbutyric acid (500 mg, 2.81 mmol) in methanol (2 mL).After heating and refluxing for 3.5 hours,The reaction solution was concentrated and dried.Then it was dissolved in dichloromethane (5 mL) and water (0.2 mL) was added sequentially.Trifluoroacetic acid (2 mL),After stirring at room temperature for 2.5 hours,The reaction solution was concentrated and dried.The resulting intermediate, 2-oxo-4-phenylbutyric acid methyl ester,It is a pale yellow oil (540 mg, 100%).This intermediate 2-methoxy-4-phenylbutyric acid methyl ester (150 mg, 0.78 mmol) was dissolved in chloroform (1 mL),A solution of bromine (1.72 mmol, 2.2 equiv) in chloroform (1 mL) was slowly added dropwise.Stir at room temperature for 24 hours.When the reaction is terminated,Diluted with ethyl acetate (30 mL),Water (5mL), after separation,The aqueous phase was extracted with ethyl acetate (3 mL x 3)Combine the organic phase,The organic phase was washed with saturated brine (3 mL).Drying with anhydrous sodium sulfate,After silica gel column chromatography (petroleum ether/ethyl acetate 12/1-8/1), 8 (97 mg) was isolated.Light yellow liquid,The yield was 36%.
References:
CN105175277,2018,B Location in patent:Paragraph 0044-0046
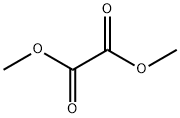
553-90-2
327 suppliers
$10.00/5g
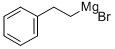
3277-89-2
17 suppliers
inquiry

83402-87-3
15 suppliers
$80.00/100mg
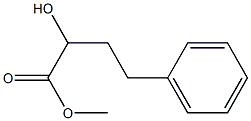
7226-82-6
1 suppliers
inquiry

83402-87-3
15 suppliers
$80.00/100mg
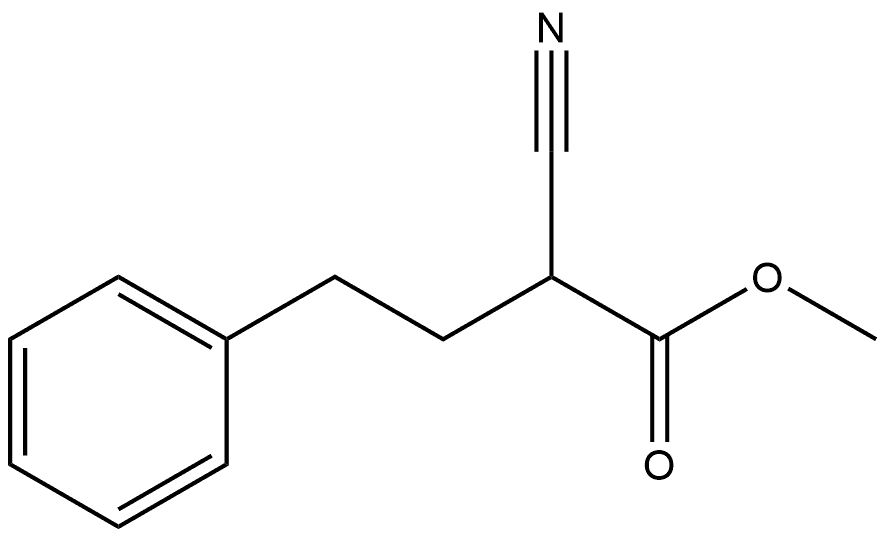
1025483-29-7
0 suppliers
inquiry

83402-87-3
15 suppliers
$80.00/100mg

553-90-2
327 suppliers
$10.00/5g
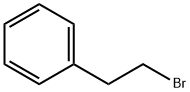
103-63-9
510 suppliers
$9.00/10g

83402-87-3
15 suppliers
$80.00/100mg