
(2-P-TOLYL-THIAZOL-4-YL)-METHANOL synthesis
- Product Name:(2-P-TOLYL-THIAZOL-4-YL)-METHANOL
- CAS Number:36093-97-7
- Molecular formula:C11H11NOS
- Molecular Weight:205.28
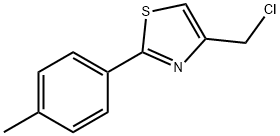
35199-18-9
20 suppliers
$45.00/50mg

36093-97-7
24 suppliers
inquiry
Yield:36093-97-7 75%
Reaction Conditions:
with sulfuric acid in water;Reflux;
Steps:
Synthesis of (2-p-tolylthiazol-4-yl)methanol (4)
Synthesis of (2-p-tolylthiazol-4-yl)methanol (4): The 4-(Chloromethyl)-2-p-tolylthiazole derivative (4.5g, 22 mmol) (3) was dissolved in 75 ml water and 75 mlconcentrated H2SO4. The reaction was refluxed for 24-48 hours and the completion was monitored by TLC.The acidic reaction was neutralized by concentratedNaOH and remain overnight at room temperature toprecipitate the product. Purification of the obtainedproduct was done by crystallization from chloroformpetroleumether.10 m.p. = 114-115 °C; Yield: 75 %; 1HNMR (400 MHz, CDCl3) δ/ppm: 2.4 (s, 3H, CH3), 3.1(brs, 1H, OH), 4.8 (s, 2H, CH2), 7.2 (d, 2H, J = 8 Hz,C3-H, C5-H phenyl), 7.27 (s, 1H, C4-H thiazole), 7.82(d, 2H, J= 8 Hz, C2-H C6-H phenyl); IR (KBr) v/cm-1:3436, 2929 (C-H stretch, aliphatic), 1649, 1454 (C=Cstretch, phenyl), 1152 (C-O, stretch), 1029, 804, 599.Elem. anal. for C11H11NOS: Calc. C: 64.36, H: 5.40, N:6.82; found C: 64.41, H: 5.36, N: 6.85.
References:
Aliabadi, Alireza;Foroumadi, Alireza;Safavi, Maliheh;Ardestani, Sussan K. [Croatica Chemica Acta,2013,vol. 86,# 3,p. 245 - 251]
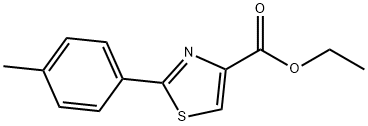
132089-32-8
52 suppliers
$75.00/100mg

36093-97-7
24 suppliers
inquiry
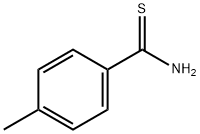
2362-62-1
95 suppliers
$14.00/250mg

36093-97-7
24 suppliers
inquiry
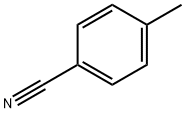
104-85-8
331 suppliers
$11.00/25g

36093-97-7
24 suppliers
inquiry