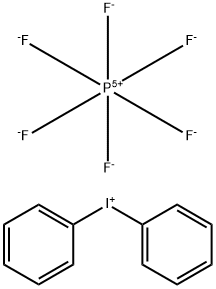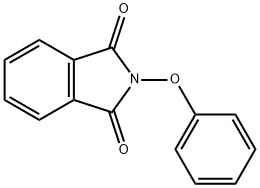
2-Phenoxyisoindoline-1,3-dione synthesis
- Product Name:2-Phenoxyisoindoline-1,3-dione
- CAS Number:64908-64-1
- Molecular formula:C14H9NO3
- Molecular Weight:239.23
Yield:64908-64-1 90%
Reaction Conditions:
with pyridine;copper chloride (I) in 1,2-dichloro-ethane at 20; for 48 h;
Steps:
6; 1
Representative Procedure for the Copper Mediated Coupling of Phenylboronic Acids and N-Hydroxyphthalimide For the Preparation of Phenoxyamines (Method 1): Synthesis of 9; A 20 mL scintillation vial was charged with N-hydroxyphthalimide (163 mg, 1.0 mmol), copper (I) chloride (99 mg, 1.0 mmol), freshly activated 4 molecular sieves (250 mg), and phenylboronic acid (244 mg, 2.0 mmol). 1,2-Dichloroethane (5 mL) was added followed by pyridine (90 μL, 1.1 mmol), resulting in a light brown suspension. The cap was loosely applied such that the reaction suspension was open to air and stirred at room temperature until completion as detected by analytical RP-HPLC (mixture color turned from brown to emerald green as the reaction proceeded). Upon completion (48 h), the mixture was adsorbed onto silica gel and concentrated to a powder. Flash chromatographic purification over silica (25% EtOAc in hexanes) afforded N-phenoxyphthalimide 9 as a white solid (216 mg, 90%); see below for characterization data.; N-Phenoxyphthalimide (9); Preparation of 9 was described above as the representative procedure (method 1). 1H-NMR (500 MHz, CDCl3) δ 7.12-7.20 (m, 3H), 7.32-7.38 (m, 2H), 7.80-7.84 (m, 2H), 7.90-7.94 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ 114.4, 124.0, 128.8, 129.7, 134.9, 158.9, 162.9; MALDI-FTMS (DHB) 240.0656 m/z [MH]+, C14H10NO3, requires 240.0655.
References:
US2006/178527,2006,A1 Location in patent:Page/Page column 7; 8; sheet 6
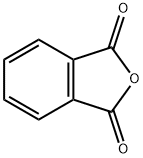
85-44-9
780 suppliers
$9.00/5g

64908-64-1
15 suppliers
inquiry