
(2-PHENYL-1-BENZOFURAN-5-YL)AMINE synthesis
- Product Name:(2-PHENYL-1-BENZOFURAN-5-YL)AMINE
- CAS Number:77084-15-2
- Molecular formula:C14H11NO
- Molecular Weight:209.24
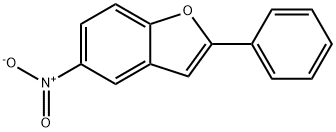
16237-97-1
0 suppliers
inquiry

77084-15-2
26 suppliers
$98.00/1g
Yield:77084-15-2 32%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in tetrahydrofuran;methanol;
Steps:
28.a
Triethylamine (63.4 ml, 0.455 mol) is added to 2-hydroxy-5-nitrobenzylphsophonium bromide (50 g, 0.101 mol) in refluxing toluene (600 ml), followed by addition of benzoyl chloride (16.2 ml, 0.140 mol) in toluene (50 ml). The mixture is refluxed for 3 hours, cooled and stirred at room temperature for 2 hours. The resulting mixture is concentrated and the product is recrystallized from 2/1 isopropyl ether/hexane, and the 2-phenyl-5-nitrobenzofuran is filtered off. This product is hydrogenated in THF (300 ml) and methanol (150 ml), with Pd/C (10%; 3.2 g). The catalyst is filtered off and the filtrate is concentrated. The residue is taken up in isopropanol/MeOH/HCl, and the hydrochloride is precipitated. It is suspended in 1M NaOH and extracted with ethyl acetate. The extracts are washed with water and concentrated. The solid is recrystallized from isopropyl ether. Yield: 9.8 g (32%); Elem. anal. C14H11NO; theory C, 80.36; H, 5.30; N, 6.70. found C, 79.78; H, 5.16; N, 6.87. IR (KBr): 3400, 3325, 1595, 1465 cm-1.
References:
US2005/197331,2005,A1 Location in patent:Page/Page column 21-22