
2-PHENYL-1 H-BENZOIMIDAZOL-4-YLAMINE synthesis
- Product Name:2-PHENYL-1 H-BENZOIMIDAZOL-4-YLAMINE
- CAS Number:313494-64-3
- Molecular formula:C13H11N3
- Molecular Weight:209.25

67152-21-0
0 suppliers
inquiry

313494-64-3
14 suppliers
$255.00/100mg
Yield:313494-64-3 79%
Reaction Conditions:
with iron;ammonium chloride in tetrahydrofuran;methanol;water at 60; for 1 h;
Steps:
1.C
Step C: 2-Phenyl-3H-benzoimidazole-4-ylamine7-nitro-2-phenyl-1H-benzoimidazole 0.137 g (0.57 mmol) obtained in Step B was dissolved in tetrahydrofuran (5 mL), methanol (5 mL) and water (5 mL). To the solution was added iron powder 0.40 g (7.2 mmol) and ammonium chloride 0.39 g (7.2 mmol), and stirred at 60° C. for 1 hour. After completion of the reaction, water was added and extracted with ethyl acetate. After drying with anhydrous magnesium sulfate, the filtrate was distilled in vacuo, and the residue was purified by column chromatography to give the title compound 0.12 g (yield: 79%).1H-NMR (500 HMz, DMSO); δ 12.5 (s, 1H), 8.10 (d, 2H), 7.557.45 (m, 3H), 6.87 (t, 1H), 6.67 (d, 1H), 6.32 (d, 1H), 5.21 (s, 2H)
References:
US2012/214804,2012,A1 Location in patent:Page/Page column 8

100-52-7
945 suppliers
$20.37/250g

313494-64-3
14 suppliers
$255.00/100mg
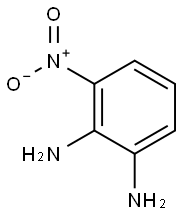
3694-52-8
157 suppliers
$15.00/250mg

313494-64-3
14 suppliers
$255.00/100mg