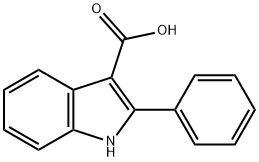
2-phenyl-1H-indole-3-carboxylic acid synthesis
- Product Name:2-phenyl-1H-indole-3-carboxylic acid
- CAS Number:59050-38-3
- Molecular formula:C15H11NO2
- Molecular Weight:237.25
Yield:59050-38-3 31%
Reaction Conditions:
with lithium tert-butoxide in N,N-dimethyl-formamide at 100; under 760.051 Torr; for 24 h;
Steps:
Representative procedure for the direct C-H carboxylation of indole derivatives 1a-p
General procedure: In a dried two-necked test tube was charged with LiOtBu (160 mg, 2.00 mmol) and indole 1a (23.4 mg, 0.4 mmol). The reaction vessel was evacuated under high vacuum and the atmosphere was replace with a balloon of CO2. Then DMF (2 mL) was added and the mixture was stirred for 24 h at 100°C. Then the result mixture was cooled and carefully quenched with a solution of HCl (2 N) and extracted with EtOAc (5x). The combined organic layers were washed with water (2x), brine (1x) and dry over MgSO4. The dried organics were concentrated under reduce pressure and the residue was purified by preparative TLC (hexane:acetone = 1:1) to afford the desired product 2a (153.0 mg, 95%) as a white solid.
References:
Yoo, Woo-Jin;Nguyen, Thanh V. Q.;Guiteras Capdevila, Montse;Kobayashi, Shu [Heterocycles,2015,vol. 90,# 2,p. 1196 - 1204]
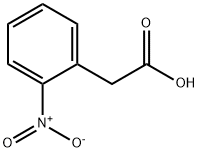
3740-52-1
247 suppliers
$9.00/10g

59050-38-3
15 suppliers
inquiry

64-18-6
1061 suppliers
$22.12/250ML
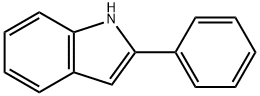
948-65-2
286 suppliers
$5.00/10g

59050-38-3
15 suppliers
inquiry
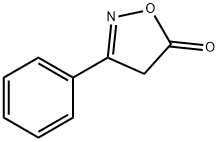
1076-59-1
93 suppliers
$27.00/1g

59050-38-3
15 suppliers
inquiry
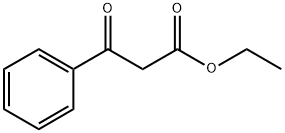
94-02-0
387 suppliers
$14.00/5g

59050-38-3
15 suppliers
inquiry
