
2-(PHENYLMETHOXY)-4-PYRIMIDINAMINE synthesis
- Product Name:2-(PHENYLMETHOXY)-4-PYRIMIDINAMINE
- CAS Number:60722-67-0
- Molecular formula:C11H11N3O
- Molecular Weight:201.22
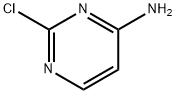
7461-50-9
273 suppliers
$10.00/1g

100-51-6
1392 suppliers
$5.00/100g

60722-67-0
38 suppliers
$37.00/100mg
Yield:-
Reaction Conditions:
Stage #1: benzyl alcoholwith sodium hydride in tetrahydrofuran at 0;Inert atmosphere;
Stage #2: 2-chloropyrimidin-4-amine in tetrahydrofuran at 0;
Steps:
6.1 Step 1: 2-(Benzyloxy)pyrimidin-4-amine
Benzyl alcohol (4.0 mL, 39.0 mmol) was added drop wise to a stirring mixture of NaN (0.36 g, 15.0 mmol) in dry THF (12 mL) at 0°C, under nitrogen atmosphere. The mixture was stirred for another 30 minutes until it forms a clear solution followed by the addition of 4-Amino-2-chloropyrimidine (0.33 g, 2.55 mmol) at 0 °C and then heated at 80 °C for 12 h. The reaction mixture was concentrated under reduced pressure. The Crude mixture was purified using column chromatography (60% EtOAc/Hexanes) to afford the desired product as a white solid. M.P. 76-78 °C (lit. 80-81 °C). ‘H NMR (400 MHz, CDCI3): 8 ppm 7.82 (d, IH), 7.36 (d, 2H), 7.25-7.2 1 (m, 3H), 5.95 (d, IH), 5.89 (hr s, 2H), 5.28 (s, 2H). ‘3C NMR (100 MHz, CDCI3); 8 ppm 165.0, 164.7, 156.5, 136.6, 128.1, 127.6, 127.5, 99.7, 68.1 ppm. IR (neat) 3465, 3324, 3034, 3034, 1635, 1559, 1455, 1352, 1292 cm1. HRMS (ESI) Calculated for C, 1H,2N30 rn/z (Mj 202.0980, Obs’d 202.0977.
References:
WO2019/13789,2019,A1 Location in patent:Page/Page column 00182; 00183