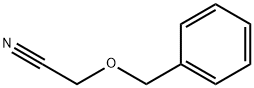
2-(Phenylmethoxy)-acetonitrile synthesis
- Product Name:2-(Phenylmethoxy)-acetonitrile
- CAS Number:13620-31-0
- Molecular formula:C9H9NO
- Molecular Weight:147.17

5774-77-6
35 suppliers
inquiry

13620-31-0
38 suppliers
inquiry
Yield: 47%
Reaction Conditions:
with trichlorophosphate in acetonitrile
Steps:
7 Example 7; Synthesis of the Protected Kaempferol (10)
Treatment of commercially available 1 (20g) with tert-butyldiphenylsilyl chloride (TBDPSCl) and imidazole in [THF/CH2C12] gave, after chromatographic purification, 2 (47.3 g, [80%).] This compound was characterized by 1H NMR. and MS. Oxidation of 2 (21.8g and 25g) using sodium chlorite gave 3 [(50G] total, quantitative yield). The product was characterized by 1H and [13C] NMR, and by MS. Benzyl alcohol [(50G)] on treatment with NaH (1.2 equiv) and ethyl bromoacetate (1 equiv) in THF gave 4a (32g, 36%), which was characterized by both [LHNMR,] and by MS. Scale up of this reaction yielded 100g of 4a. Reaction [OF 4A (5G)] with NH40H at [0° FOR] 5 h in [CH2C12] gave amide 4b (4.3g, 96%), which was characterized by 1H NMR and MS. A repeat of this experiment on 45 g [OF 4A] gave 38 g (94%) [OF 4B.] Dehydration of 4b (4.2 g) using [POC13] in acetonitrile gave 5 (1.75 g, 47%), which was characterized by 1H NMR, [13C] NMR and MS. A repeat of this experiment on 38g of 4b gave an additional 15.75 g (47%) of 5. Coupling of 5 (5 g) and phloroglucinol in MTBE with [HC1] gas bubbling at [0°C] for 3 h gave 6 (2.6 g, 56%), which was characterized [BY IH] NMR, 13C NMR and MS. Selective protection of 6 (0.5 g) using TBDPSCI (2.5 equiv) and Et3N (2.5 equiv) in [CH2C12] at room temperature for 16 h gave 7 (1.2 g, 85%), which was characterized [BY LH] NMR and MS. Scale-up of this experiment on 2 g of 6 gave an additional 3.4 [G] [(62%)] of 7. Condensation of 7 (1.4 g) with 3 (1.35 equiv) in [CH2C12] [EDCI (1.5 equiv), DMAP (0. [35] equiv), [TSOH] (0. [35] equiv. ] at room temperature for 24 h gave 8 (1.5 g, 72%), which was characterized by 1H NMR. Scale up gave 35g of purified 8. Compound 8 (6g) was debenzylated using Rh/C as a catalyst (H2, 60 psi, EtOAc, rt, 24h) to give 8a (1.8g, 33%) along with 2.9g (53%) of the trans-esterified (migration of benzoyl group [RI)] product 8b. Both the intermediates 8a and 8b were characterized by 1H NMR
References:
University of Virginia Patent Foundation WO2003/105766, 2003, A2 Location in patent:Page 46-47
60656-87-3
174 suppliers
$17.00/1g

13620-31-0
38 suppliers
inquiry
100-51-6
1390 suppliers
$5.00/100g

13620-31-0
38 suppliers
inquiry
32122-09-1
105 suppliers
$15.00/1g

13620-31-0
38 suppliers
inquiry
