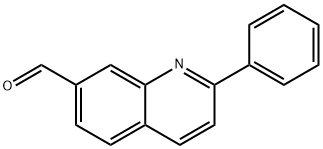
2-phenylquinoline-7-carbaldehyde synthesis
- Product Name:2-phenylquinoline-7-carbaldehyde
- CAS Number:867162-43-4
- Molecular formula:C16H11NO
- Molecular Weight:233.26
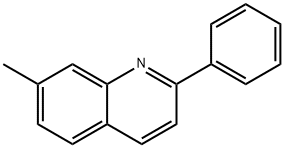
27356-39-4
17 suppliers
inquiry

867162-43-4
29 suppliers
$189.00/250mg
Yield:-
Reaction Conditions:
with selenium(IV) oxide at 160; for 22 h;Product distribution / selectivity;
Steps:
1
2-Phenylquinoline-7-carbaldehyde; [1070] A mixture of 7-methyl-2-phenylquinoline (2.49g, 11.4mmol) and selenium dioxide (1.92g, 17.3mmol, 1.5eq.) is heated to 160°C (bath temp. ) for 22h. The cooled melt is suspended in CH2C12 with the aid of sonication and filtered through Celite and then through a plug of silica gel. This effectively removes the red color and the major lower spots. The material thus obtained is crystallized from hexanes/CHCl3, yielding a pale beige solid, mp. 108°C. The mother liquor is concentrated and chromatographed on silica gel [Jones Flashmaster, 50g /150mL cartridge, eluting with hexanes:CH2C12 1: 1 (1-25) No. 1: 3 (26-53) No. CH2C12 (54-73) No.3%. EtOAc in CH2C12 (74-85) ] to obtain as pale yellow solid, mp. 109°C. 'H NMR (CDCl3, 400MHz) 5 7.48-7.60 (m, 3H), 7.94 (d, J= 8.8 Hz, 1H), 8.01-8.05 (m, 2H), 8.18-8.23 (m, 2H), 8.29 (d, J= 8.8 Hz, 1H), 8.64 (s, 1H), 10.26 (s, 1H). MS (ES+): m/z 234.2 (100) [MH+]. HPLC: tR = 3.0 min (MicromassZQ, nonpolar_5min); 13C NMR (CDC13, 100.6 MHz, DEPT135) 8 121.22 (+), 122.80 (+), 127.51 (2C, +), 128.65 (+), 128.94 (2C, +), 129.83 (+), 130.69 (Cquart), 135.84 (+), 136.68 (+), 137.21 (Cquart), 138.79 (Cquart), 147.91 (Cquart), 158.48 (Cquart), 192.14 (+) ; IR (film) : v = 3059 cm-1,3034, 2824, 2717, 1954, 1812,1684, 1601,1554, 1510,1491, 1448,1420, 1392, 1320,1280, 1168, 1145, 1120, 1075, 1052, 1025, 971, 926, 897, 850, 812, 787, 757, 692, 673, 627.
References:
WO2005/97800,2005,A1 Location in patent:Page/Page column 222-223
361457-37-6
22 suppliers
inquiry

867162-43-4
29 suppliers
$189.00/250mg
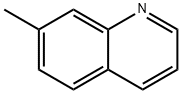
612-60-2
158 suppliers
$30.00/5g

867162-43-4
29 suppliers
$189.00/250mg
591-51-5
128 suppliers
$123.00/50ml

867162-43-4
29 suppliers
$189.00/250mg