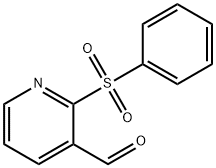
2-(Phenylsulfonyl)Nicotinaldehyde(WXC03058) synthesis
- Product Name:2-(Phenylsulfonyl)Nicotinaldehyde(WXC03058)
- CAS Number:1161864-92-1
- Molecular formula:C12H9NO3S
- Molecular Weight:247.27
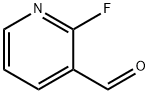
36404-90-7
164 suppliers
$10.00/1g
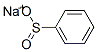
873-55-2
384 suppliers
$10.00/5g

1161864-92-1
7 suppliers
inquiry
Yield:1161864-92-1 51%
Reaction Conditions:
in dimethyl sulfoxide at 100; for 94 h;
Steps:
1 2-(Benzenesulfonyl)isonicotinaldehyde
2-(Benzenesulfonyl)isonicotinaldehyde To a stirred suspension of benzenesulfinic acid sodium salt (9.36 g, 0.057 mol) in DMSO (45 ml), was added 2-fluoro-3-pyridinecarboxaldehyde (5.20 ml, 0.052 mol). The resulting mixture was stirred at 100° C. for 94 hours. After cooling to room temperature, the reaction mixture was partitioned between ethyl acetate and water. The organic layer was separated and the aqueous further extracted with ethyl acetate (3*150 ml). The combined organic extracts were washed with water (100 ml) and brine (100 ml), dried over anhydrous MgSO4, filtered and evaporated. The resulting solid was purified by column chromatography eluding with ethyl acetate:hexanes 0:100 to 60:40 v/v to afford 6.56 g (51%) of the title compound (LCMS RT=5.63 min, MH+ 248). 1H NMR (DMSO): 10.89 (1H, d, J=0.68 Hz), 8.82 (1H, dd, J 1.7, 4.7 Hz), 8.32 (1H, dd, J 1.7, 7.9 Hz), 8.08-8.02 (2H, m), 7.85 (1H, dd, J 7.9, 0.7 Hz), 7.81 (1H, dt, J 1.3, 7.5 Hz), 7.73-7.65 (2H, m).
References:
US2009/186923,2009,A1 Location in patent:Page/Page column 8