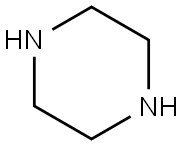
2-PIPERAZIN-1-YLMETHYL-1H-BENZOIMIDAZOLE synthesis
- Product Name:2-PIPERAZIN-1-YLMETHYL-1H-BENZOIMIDAZOLE
- CAS Number:59052-85-6
- Molecular formula:C12H16N4
- Molecular Weight:216.28
Yield:59052-85-6 47%
Reaction Conditions:
with potassium carbonate in ethanol;
Steps:
4.2. General procedure for the synthesis of compounds 6a~6l and 9a~9l
General procedure: The synthetic routes to final compounds 6a~6l and 9a~9l were depicted in Scheme 1. First, 2,4,6-Trichloro-1,3,5-triazine (1.1 equiv) and K2CO3 (1.1 equiv) were dissolved in THF(Dissolve) at -20 °C, [26-28], and then the secondary amine (1.0 equiv) was dissolved in THF, dilute about 20 times, add dropwise the secondary amine solution in the triazine solution to start the reaction. The TLC plate detects the progress of the reaction. After the reaction is completed, the reaction system is extracted with a mixed solution of CH3OOC2H5/H2O and CH3OOC2H5/NaHCO3 to obtain a monosubstituted 1,3,5-triazine compound (2a-2l). Followed by, 2-chloro-1H-benzo[d]imidazole (3) (1.0 equiv) and piperazine (4) (1.5 equiv) in 1,4-dioxane, and reacting at 110 °C in the presence of Cs2CO3 (1.0 equiv), and then purified by column chromatography (Dichloromethane/methanol=20:1) to obtain pure product of 2-(piperazin-1-yl)-1H-benzo[d]imidazole (5) [29]. 2-(chloromethyl)-1H-benzo[d]imidazole (7) (1.0 equiv) reacted with piperazine (4) (1.2 equiv) in ethanol at 86 °C in the presence of K2CO3 (1.1 equiv), and then purifying by column chromatography (Dichloromethane/methanol=10:1) to get pure product of 2-(piperazin-1-ylmethyl)-1H-benzo[d]imidazole (8). Finally, compound 5 and compound 8 were respectively reacted with monosubstituted 1,3,5-triazine in 1,4-dioxane to obtain the targeted compounds 6a~6l and 9a~9l. All the newly synthesized compounds were stable enough to be isolated by column chromatography (Ethyl acetate/petroleum ether) on silicagel and their structures were unambiguously verified by different analysis and spectroscopic data. e. g. 1H-NMR, 13C-NMR, HRMS, FT-IR and XRD.
References:
Liu, Shan-Ming;Liu, Wei-Wei;Liu, Yu-Han;Qian, Jing-Jing;Qin, Tian;Shi, Da-Hua;Shi, Li-Ying;Wang, Jing;Wen, Ze-Yu;Wu, Wen-Long;Yang, Qun;Yang, Shun;Zou, Jing-Pei [Journal of Molecular Structure,2022,vol. 1257,art. no. 132498]
4857-04-9
203 suppliers
$6.00/1g

59052-85-6
14 suppliers
$378.00/1g
95-54-5
501 suppliers
$9.00/1g

59052-85-6
14 suppliers
$378.00/1g