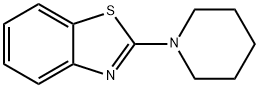
2-piperidinobenzothiazole synthesis
- Product Name:2-piperidinobenzothiazole
- CAS Number:2851-08-3
- Molecular formula:C12H14N2S
- Molecular Weight:218.32

2762-59-6
9 suppliers
inquiry

2851-08-3
13 suppliers
$28.60/25MG
Yield:2851-08-3 174 mg
Reaction Conditions:
with oxygen;copper(II) bis(trifluoromethanesulfonate) in toluene; for 12 h;Reflux;
Steps:
4.2 General procedure for preparation of substituted 2-morpholinobenzo[d]thiazoles from substituted N-(phenyl) morpholine-4-carbothioamide using Cu(OTf)2 (1-10)
General procedure: Substituted phenyl isothiocyanate (1-10) (1.5mmol) was treated with morpholine a (1.5mmol) under neat condition and instant formation of the corresponding thiourea was observed. To this in situ generated thiourea toluene (2mL) was added and medium was stirred at room temperature for 5min followed by the addition of copper triflate (10mol%). After that the reaction mixture was subjected to reflux in a preheated oil bath at 110°C under O2 atmosphere. The progress of the reaction was monitored by TLC. After the completion of the reaction as judged from TLC the reaction mixture was cooled and then admixed with water (1mL) and the product was extracted with ethyl acetate (2×10mL). The organic layer was dried over anhydrous sodium sulfate (Na2SO4), filtered and evaporated under reduced pressure. The crude products so obtained were further purified through silica gel column chromatograph (hexane/ethyl acetate, 9:1) to yield the pure substituted 2-morpholinobenzo[d]thiazole (1a′-10a′). The identity and purity of the product was further confirmed by spectroscopic analysis. Reaction performed with phenyl isothiocyanate (1) (1.5mmol) and other secondary amines (b-f) (1.5mmol) in the above mentioned method and corresponding benzo[d]thiazoles (1b′-f′) were isolated and spectroscopically characterized.
References:
Banerjee, Arghya;Santra, Sourav Kumar;Rout, Saroj Kumar;Patel, Bhisma K. [Tetrahedron,2013,vol. 69,# 43,p. 9096 - 9104]

110-89-4
0 suppliers
$15.96/100ML
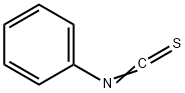
103-72-0
245 suppliers
$10.00/1g

2851-08-3
13 suppliers
$28.60/25MG

110-89-4
0 suppliers
$15.96/100ML

75-15-0
259 suppliers
$40.00/100g
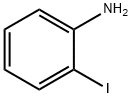
615-43-0
425 suppliers
$5.00/5g

2851-08-3
13 suppliers
$28.60/25MG

110-89-4
0 suppliers
$15.96/100ML
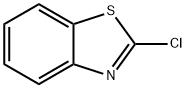
615-20-3
245 suppliers
$19.00/25g

2851-08-3
13 suppliers
$28.60/25MG
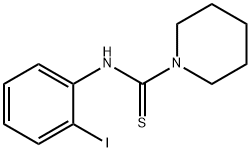
1224507-79-2
0 suppliers
inquiry

2851-08-3
13 suppliers
$28.60/25MG