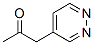
2-Propanone, 1-(4-pyridazinyl)- (9CI) synthesis
- Product Name:2-Propanone, 1-(4-pyridazinyl)- (9CI)
- CAS Number:125375-25-9
- Molecular formula:C7H8N2O
- Molecular Weight:136.1512
Yield:125375-25-9 2 g
Reaction Conditions:
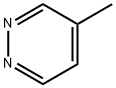
Stage #1: 4-methylpyridazinewith lithium diisopropyl amide in tetrahydrofuran at -78; for 0.5 h;
Stage #2: acetic anhydride in tetrahydrofuran at -65 - 20;
Steps:
12.1 Example 12: Preparation of 3-methyl-4-pyridazin-4-yl-lH-pyrrole-2-carboxylic acid amide Step 1: Preparation of l-pyridazin-4-yl-propan-2-one
To a solution of 4-methyl-pyridazine (3.76 g, 40 mmol) in THF (40 mL) was added LDA (24 mL, 0.048 mol, 2.0 M) dropwise at -78 °C. After being stirred at -78 °C for 30 minutes, acetic anhydride (4.9 g, 0.048 mol) was added dropwise below -65 °C. The resulting mixture was warmed to room temperature slowly. The reaction was quenched with saturated NH4C1 solution (10 mL), and then dried over anhydrous Na2S04 and concentrated. The residue was purified by flash chromatography to give l-pyridazin-4-yl-propan-2-one (2 g) as yellow oil.
References:
WO2014/154723,2014,A1 Location in patent:Page/Page column 47