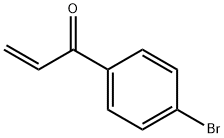
2-Propen-1-one, 1-(4-bromophenyl)- synthesis
- Product Name:2-Propen-1-one, 1-(4-bromophenyl)-
- CAS Number:22731-70-0
- Molecular formula:C9H7BrO
- Molecular Weight:211.06
Yield:22731-70-0 95%
Reaction Conditions:
with trifluoroacetic acid;diisopropylamine 2,2,2-trifluoroacetic acid salt in tetrahydrofuran; for 2 h;Reflux;
Steps:
General Procedure for the α-Methylenation
General procedure: To a solution of a carbonyl compound (1.0mmol) and paraformaldehyde (2.0 mmol) in dry THF (1.0 mL) was added diisopropylammoniumtrifluoroacetate (1.0 mmol) and trifluoroacetic acid (0.1 mmol). The reaction mixture was stirredat reflux for 2 h, then cooled down to room temperature and a second addition ofparaformaldehyde (2.0 mmol) was performed. Next, the reaction mixture was stirred at refluxovernight. Then cooled down and the solvent was removed under reduced pressure, dissolved inEt2O and washed with 1N HCl, 1N NaOH, and brine. The result solution was dried over Na2SO4and concentrated under vacuum. The crude product was purified by silica gel columnchromatography to give the product.
References:
Guo, Shi-Huan;Xing, Sheng-Zhu;Mao, Shuai;Gao, Ya-Ru;Chen, Wen-Liang;Wang, Yong-Qiang [Tetrahedron Letters,2014,vol. 55,# 49,p. 6718 - 6720] Location in patent:supporting information
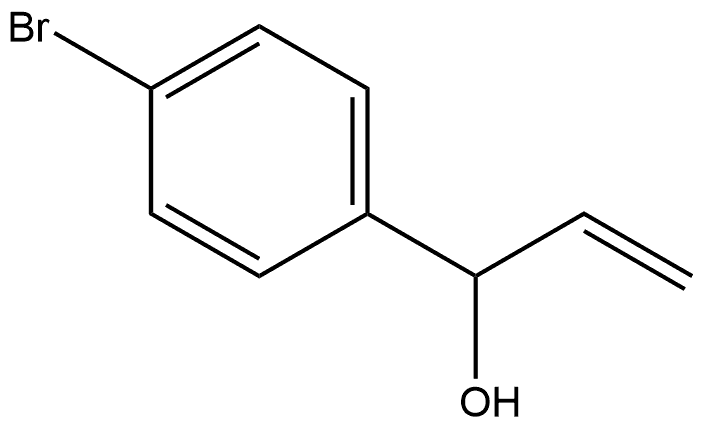
58824-56-9
3 suppliers
inquiry

22731-70-0
9 suppliers
inquiry
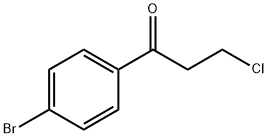
31736-73-9
161 suppliers
$9.00/5g

22731-70-0
9 suppliers
inquiry