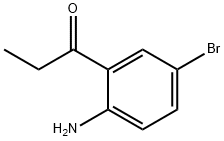
2-propionyl-4-bromoaniline synthesis
- Product Name:2-propionyl-4-bromoaniline
- CAS Number:124623-15-0
- Molecular formula:C9H10BrNO
- Molecular Weight:228.09
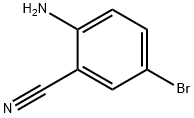
39263-32-6
242 suppliers
$15.00/1g
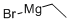
925-90-6
346 suppliers
$12.00/5ml

124623-15-0
16 suppliers
inquiry
Yield:124623-15-0 76%
Reaction Conditions:
Stage #1: 2-amino-5-bromobenzonitrile;ethynylmagnesium bromide in tetrahydrofuran; for 2 h;Cooling with ice;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;water monomer; for 2 h;
Steps:
53.1 1-(2-Amino-5-bromophenyl)propan-1-one 53b
2-Amino-5-bromobenzonitrile 53a (500 mg, 2.54 mmol, prepared according to the known method disclosed in "European Journal of Medicinal Chemistry, 2014, 76, 341-343") was dissolved in 10 mL of tetrahydrofuran. The reaction solution was cooled in an ice bath, added dropwise with 12.69 mL of 1.0 M ethyl magnesium bromide under an argon atmosphere, and stirred for 2 hours. The reaction solution was added with 6 M hydrochloric acid, and stirred for 2 hours. The reaction solution was added with saturated sodium carbonate solution, and extracted with ethyl acetate (50 mL×3). The organic phases were combined, washed with saturated sodium chloride solution, dried over sodium sulfate, and filtrated. The filtrate was concentrated under reduced pressure, and the residue was purified by CombiFlash rapid preparation instrument with elution system B to obtain the title compound 53b (440 mg), yield: 76.02%.
References:
EP3569596,2019,A1 Location in patent:Paragraph 0287; 0288

106-40-1
458 suppliers
$12.00/5g

124623-15-0
16 suppliers
inquiry

811-49-4
57 suppliers
$140.00/4x25ml
5794-88-7
465 suppliers
$6.00/10g

124623-15-0
16 suppliers
inquiry
![N-[4-Bromo-2-(1-oxopropyl)phenyl]acetamide](/CAS/20210305/GIF/188256-42-0.gif)
188256-42-0
0 suppliers
inquiry

124623-15-0
16 suppliers
inquiry
