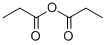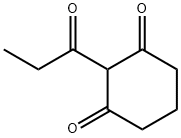
2-propionylcyclohexane-1,3-dione synthesis
- Product Name:2-propionylcyclohexane-1,3-dione
- CAS Number:104775-30-6
- Molecular formula:C9H12O3
- Molecular Weight:168.19
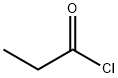
79-03-8
404 suppliers
$9.00/1g

504-02-9
554 suppliers
$5.00/10g
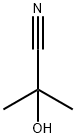
75-86-5
2 suppliers
$64.90/5G

104775-30-6
2 suppliers
inquiry
Yield:104775-30-6 84%
Reaction Conditions:
with triethylamine in dichloromethane;water;
Steps:
8 Production of 2-Propanoyl-1,3-cyclohexanedione
EXAMPLE 8 Production of 2-Propanoyl-1,3-cyclohexanedione To a mixture of 3.0 g (0.027 mole) of 1,3-cyclohexanedione and 3.8 ml (0.027 mole) of triethylamine in 15 ml methylene chloride, there was added dropwise 2.3 ml (0.027 mole) of propionyl chloride with stirring and cooling in a room temperature water bath. After continued stirring at ambient temperature for about 4 hours, an additional 7.5 ml (0.054 mole) of triethylamine and 0.25 ml (10 mole percent with respect to enol ester) of acetone cyanohydrin were added. The mixture was stirred at ambient temperature overnight, and was then diluted with water and acidified with 6N hydrochloric acid. The phases were separated, and the aqueous phase was extracted with methylene chloride. The combined organic phases were dried over anhydrous sodium sulfate and concentrated under reduced pressure to give 4.68 g of crude product as a mixture of solid and liquid. The crude product was dissolved in methylene chloride and was extracted with 2.5N sodium hydroxide solution followed by water. The combined aqueous phases were acidified with 6 N hydrochloric acid and extracted with methylene chloride. The organic extract was dried over anhydrous sodium sulfate and concentrated under reduced pressure to give 3.83 g of oily product (84% of theoretical). Structure of the product was confirmed by infrared, nuclear magnetic resonance and mass spectroscopy.
References:
US4695673,1987,A