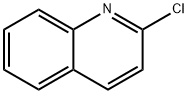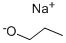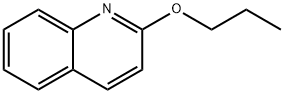
2-Propoxyquinoline synthesis
- Product Name:2-Propoxyquinoline
- CAS Number:945-83-5
- Molecular formula:C12H13NO
- Molecular Weight:187.24
Yield:945-83-5 96%
Reaction Conditions:
in propan-1-ol; for 4 h;Reflux;
Steps:
5.1.1. 2-Propoxyquinoline 3
2-Chloroquinoline 1 (500 mg, 3.06 mmol) was added to a solution of sodium n-propoxide (10.1 mmol prepared from 232 mg of Na) in nPrOH (10 mL). The mixture was refluxed for 4 h. The solvent was removed, water was added and the mixture was extracted with EtOAc. After drying over MgSO4, the solvent was removed to give compound 3 (551 mg, 2.94 mmol, 96%) as a colorless oil.CommentIR (ATR): 1618, 1606, 1428, 1313, 1276, 1259, 1238 cm-1.Comment1H NMR (400 MHz, DMSO-d6): δ 1.01 (3H, t, J = 7.5 Hz), 1.79 (2H, sex, J = 7 Hz), 4.36 (2H, t, J = 6.5 Hz), 7.00 (1H, d, J = 9 Hz), 7.41 (1H, t, J = 7.5 Hz), 7.65 (1H, t, J = 7.5 Hz), 7.87 (1H, d, J = 8 Hz), 7.75 (1H, d, J = 8 Hz), 8.22 (1H, d, J = 9 Hz).Comment13C NMR (100 MHz, DMSO-d6): δ 10.5 (CH3), 21.8, 66.9 (CH2), 113.0, 124.0, 126.7, 127.7, 129.7, 139.2 (CHarom), 124.7, 145.9, 161.7 (Carom).CommentHRMS (ESI+): m/z calcd for C12H14NO (M+H)+ 188.1075. Found 188.1077.
References:
Saugues, Emmanuelle;Nauton, Lionel;Thery, Vincent;Anizon, Fabrice;Moreau, Pascale [Bioorganic Chemistry,2011,vol. 39,# 4,p. 143 - 150] Location in patent:experimental part
71-23-8
749 suppliers
$12.00/100g
1613-37-2
4 suppliers
$54.31/5G

945-83-5
16 suppliers
$60.00/100mg