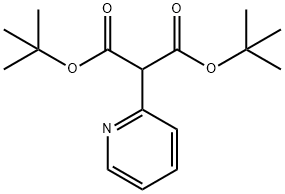
2-Pyridin-2-yl-Malonic acid di-tert-butyl ester synthesis
- Product Name:2-Pyridin-2-yl-Malonic acid di-tert-butyl ester
- CAS Number:1104643-39-1
- Molecular formula:C16H23NO4
- Molecular Weight:293.36
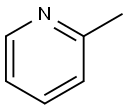
109-06-8
397 suppliers
$16.00/25g

24424-99-5
822 suppliers
$13.50/25G

1104643-39-1
13 suppliers
$450.00/2.5 G
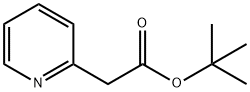
150059-62-4
20 suppliers
$150.00/250mg
Yield: 29%
Reaction Conditions:
Stage #1:α-picoline with n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at -78; for 1 h;
Stage #2:di-tert-butyl dicarbonate in tetrahydrofuran at -78 - 20; for 2 h;
Steps:
14.1 Step 1:
To a stirred solution of diisopropylamine (10.8 g, 0.1 mol) in (20 ml.) of dry tetrahydrofuran was added n-BuLi (49 ml_, 2.04M, 0.10 mol) at -78 °C. The reaction mixture was allowed to stir for 30 min. To this solution, 2-methylpyridine (10 g, 0.107 mol) in (20 mL) of dry tetrahydrofuran was added dropwise. The reaction mixture was allowed to stir for 1 h at -78 °C. To this di-ferf-butyl dicarbonate (24 g, 0.11 mol) was added at -78 °C and was allowed to attain room temperature in 2 h. The reaction mixture was quenched with saturated ammonium chloride solution (50 mL), diluted with water (60 mL) and extracted with ethyl acetate (3 x 80 mL).The total organic layer was washed with brine (50 mL).The final organic layer was dried over anhydrous magnesium sulfate and was concentrated under reduced pressure to obtain crude compound which was purified by column chromatography (silica gel: 100-200 mesh, eluent: 10% ethyl acetate in n-hexane) to afford tert-butyl 2-(pyridin-2- yl)acetate (6 g, 29 %).
References:
GRÜNENTHAL GMBH;FRANK, Robert;CHRISTOPH, Thomas;LESCH, Bernhard;LEE, Jeewoo WO2013/13817, 2013, A1 Location in patent:Page/Page column 92

109-06-8
397 suppliers
$16.00/25g

24424-99-5
822 suppliers
$13.50/25G

1104643-39-1
13 suppliers
$450.00/2.5 G