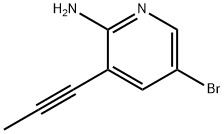
2-PyridinaMine, 5-broMo-3-(1-propyn-1-yl)- synthesis
- Product Name:2-PyridinaMine, 5-broMo-3-(1-propyn-1-yl)-
- CAS Number:1312755-43-3
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
Yield:1312755-43-3 93%
Reaction Conditions:
with triethylamine;bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide in tetrahydrofuran at 0 - 20; for 1 h;
Steps:
Step 2 : 5-Bromo-3-prop-l-ynyl-pyridin-2-ylamine Propyne (28 g, 700 mmol) was condensed into a pre- weighed flask cooled to ca. -40 °C containing THF (150 mL). The solution was added via cannula to a cooled (0-5 °C), degassed mixture of 5-bromo-3-iodo-pyridin-2-ylamine (139 g, 465 mmol),bi5'(triphenylphosphine)dichloropalladium(0) (16.3 g, 23.2 mmol), copper (I) iodide (5.3 g, 27.9 mmol) and triethylamine (141 g, 194 mL, 1.4 mol) in THF (1.25 L). The mixture was stirred at 0-5 °C for 30 minutes then for a further 30 minutes at ambient temperature. The solid was removed by filtration and the cake washed with THF. The filtrate was diluted with ethyl acetate and extracted with 2M hydrochloric acid (x 3). The combined acid extract was washed with diethyl ether and then made basic by careful addition of potassium carbonate then extracted with diethyl ether (x 3). The combined organic layer was dried (Na2S04), filtered and evaporated to afford the title compound as a buff solid(91 g, 93%). NMR (400 MHz, CDC13): 8.01 (d, J = 2.4 Hz, 1H), 7.55 (d, J = 2.4 Hz, 1H), 4.96 (s, 2H), 2.11 (s, 3H).
References:
WO2011/73263,2011,A1 Location in patent:Page/Page column 78-79
6224-91-5
253 suppliers
$12.00/1g

381233-96-1
315 suppliers
$8.00/1g

1312755-43-3
1 suppliers
inquiry

1072-97-5
708 suppliers
$9.00/1g

1312755-43-3
1 suppliers
inquiry
