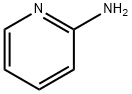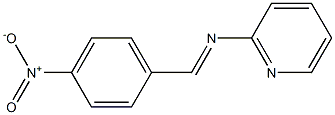
2-Pyridinamine, N-[(4-nitrophenyl)methylene]- synthesis
- Product Name:2-Pyridinamine, N-[(4-nitrophenyl)methylene]-
- CAS Number:5918-77-4
- Molecular formula:C12H9N3O2
- Molecular Weight:227.2188
Yield:5918-77-4 85%
Reaction Conditions:
with acetic acid in methanol; for 3 h;Reflux;
Steps:
3.2. General Procedure for the Synthesis of Compounds 1-12
General procedure: An equal molar solution of a primary amine (0.01 mol) and an aldehyde (0.01 mol) in dry methanol(50 mL) was refluxed for 3 h using a few drops of acetic acid as a catalyst. The reaction mixture wasthen cooled to room temperature and the formed precipitate was filtered, washed with methanol 35 mL) and diethyl ether (2 5 mL), and then dried under vacuum. The recrystallization frommethanol/dioxane (1:4) mixture gave pure products (1-12).
References:
Toubi, Yahya;Abrigach, Farid;Radi, Smaail;Souna, Faiza;Hakkou, Abdelkader;Alsayari, Abdulrhman;Muhsinah, Abdullatif Bin;Mabkhot, Yahia N. [Molecules,2019,vol. 24,# 18,art. no. 3250]
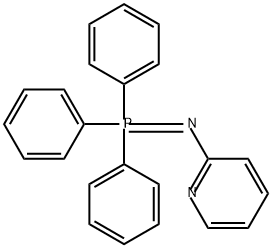
23574-81-4
0 suppliers
inquiry

555-16-8
530 suppliers
$5.00/5g
![2-Pyridinamine, N-[(4-nitrophenyl)methylene]-](/CAS/20180527/GIF/5918-77-4.gif)
5918-77-4
0 suppliers
inquiry
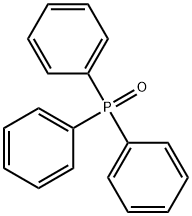
791-28-6
494 suppliers
$6.00/25g