2-Pyridinecarboxamide,4-methyl-(9CI) synthesis
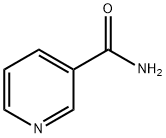
- Product Name:2-Pyridinecarboxamide,4-methyl-(9CI)
- CAS Number:54089-04-2
- Molecular formula:C7H8N2O
- Molecular Weight:136.15
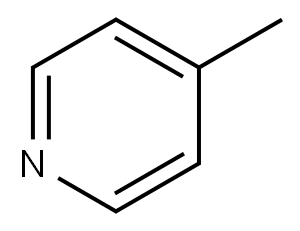
108-89-4
332 suppliers
$16.00/25mL

60100-09-6
0 suppliers
inquiry

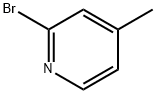
54089-04-2
17 suppliers
$240.00/1g
Yield:54089-04-2 89%
Reaction Conditions:
with dipotassium peroxodisulfate;oxygen;sodium formate;silver nitrate in water at 80; for 3 h;Schlenk technique;regioselective reaction;
Steps:
general procedure for Ag-catalyzed carbamoylation
General procedure: A 25 mL Schlenk flask was charged with AgNO3 (17.2 mg, 20 mol% Ag), K2S2O8(408 mg, 1.5 mmol), and HCOONa (129 mg, 1.0 mmol) before standard cycles of evacuation and back-filling with dry and pure oxygen (three times). Corresponding pyridine 1 (0.5 mmol), formamide 2 (2 mL), and H2O (0.4 mL) were added successively. The mixture was stirred at 80 °C for the indicated time (monitored byTLC). At the end of the reaction, the reaction mixture was cooled to room temperature, poured into a saturated aqueous NaCl solution (15 mL), and extracted with ethyl acetate (3 × 15 mL). The organic phases were combined, and the volatile components were evaporated in a rotary evaporator. The residue was purified by flash column chromatography on silica gel to afford the corresponding product 3
References:
Han, Wei;Jin, Fengli;Zhao, Qian;Du, Hongyan;Yao, Lifang [Synlett,2016,vol. 27,# 12,art. no. ST-2016-W0059-L,p. 1854 - 1859] Location in patent:supporting information
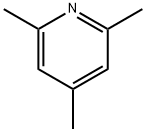
108-75-8
457 suppliers
$5.00/10g

100-48-1
507 suppliers
$8.00/25g
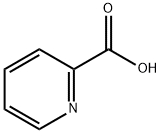
98-98-6
625 suppliers
$5.00/10g

54089-04-2
17 suppliers
$240.00/1g

54089-05-3
5 suppliers
inquiry