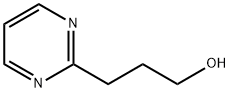
2-Pyrimidinepropanol (9CI) synthesis
- Product Name:2-Pyrimidinepropanol (9CI)
- CAS Number:260441-09-6
- Molecular formula:C7H10N2O
- Molecular Weight:138.17

260441-08-5
21 suppliers
inquiry

260441-09-6
15 suppliers
inquiry
Yield:260441-09-6 91%
Reaction Conditions:
with hydrogen;10% palladium on activated carbon in ethyl acetate; for 6 h;
Steps:
To a solution of 2-Bromopyrimidine (7.95 g, 0.05 M) in acetonitrile (150 mL) was added propargylalcohol (4.2 g, 0.075 M ), bis-(triphenylphosphine)-palladium(11)chloride (750 mg, 1 mM), copper iodide (100 mg, 0.5 mM) and triethylamine (25 mL, 0.25 M) and the mixture was stirred and heated at 70° C. for 2 hours. An additional amount of propargyl alcohol (2.1 g, 0.038 M), bis-(triphenylphosphine)-palladium(11)chloride (375 mg, 0.5 mil), and copper iodide (50 mg, 0.25 mil) was then added to the reaction mixture which was stirred and heated at 70° C. for an additional 1 hour. [00092] The reaction mixture was evaporated to dryness and the residue which was pre-adsorbed on to silica, chromatographed. Elution with ethyl acetate gave 3-(2-pyrimidyl) prop-2-yn-3-ol as a yellow solid 4.45 g (66%). NMR (CDCl3) 2.9 (1H, t), 4.5 (2H, d), 7.3 (1H, d), 8.8 (2H, t), MS found MH+ 135. [00093] 3-(2-pyrimidyl)propan-1-ol (4.45 g; 0.033 M) was dissolved in ethyl acetate (140 mL), 10% Pd/C (890 mg) was added and the mixture stirred under an atmosphere of hydrogen for 6 hours. The reaction mixture was filtered through Celite and the filtrate evaporated to give 3-(2-pyrimidyl)propan-1-ol as a yellow oil, 4.15 g (91%). NMR (CDCl3) 2.1 (2H, m), 3.2 (2H, t), 3.8 (2H, t), 7.2 (1H, t), 8.7 (2H, d) MS found MH+ 139. [00094] 3-(2-pyrimidyl)propan-1-ol was oxidized to give 3-(2-pyrimidyl) propionaldehyde as a yellow oil NMR (CDCl3) 3.0 (2H, t), 3.4 (2H, t), 7.1 (1H, t), 8.7 (2H, d), 9.9 (1H, s) using the Swern oxidation described in this patent.
References:
US6734184,2004,B1 Location in patent:Page/Page column 17
![Pyrimidine, 2-[3-[(tetrahydro-2H-pyran-2-yl)oxy]propyl]-](/CAS/20210305/GIF/374601-09-9.gif)
374601-09-9
0 suppliers
inquiry

260441-09-6
15 suppliers
inquiry
![Pyrimidine, 4,6-dichloro-2-[3-[(tetrahydro-2H-pyran-2-yl)oxy]-1-propyn-1-yl]-](/CAS/20210305/GIF/374601-07-7.gif)
374601-07-7
0 suppliers
inquiry

260441-09-6
15 suppliers
inquiry