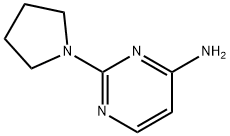
2-(pyrrolidinyl)pyrimidin-4-amine synthesis
- Product Name:2-(pyrrolidinyl)pyrimidin-4-amine
- CAS Number:33851-99-9
- Molecular formula:C8H12N4
- Molecular Weight:164.21
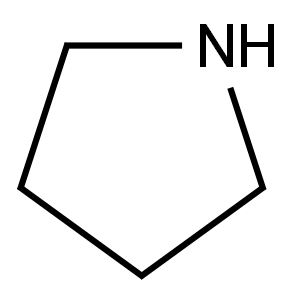
123-75-1
533 suppliers
$10.00/5g
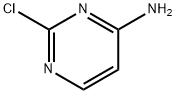
7461-50-9
266 suppliers
$10.00/1g

33851-99-9
21 suppliers
$185.00/1g
Yield:33851-99-9 100%
Reaction Conditions:
at 120; for 1.5 h;
Steps:
103.1 Step 1. 2-(Pyrrolidiii-l-yl)pyrimidin-4-amine
In a 20 ml vial was added a suspension of 4-amino-2-chloropyrimidine (1.5 g, 11.58 mmol) and pyrrolidine (4.79 ml, 57.9 mmol). The mixture was heated in a heating block at 120 °C for 1.5 h. After cooling to room temperature, the reaction mixture was diluted with DCM and concentrated. The residue was diluted with DCM and washed with aq NHUQH solution and reconcentrated to obtain 2-(pyrrolidin-l-yl)pyrimidin-4-amine (1.9 g, 11.57 mmol, 100 % yield) as a tan-yellow solid. 1H NMR (400MHz, DMSQ-db) d 7.70 (d,.1 5.6 Hz, i l l). 6.28 (br. s., 2H), 5.68 (d,.1 5.7 Hz, 11 1). 3.41 - 3.35 (m, 4H), 1.88 - 1.83 (m, 41 1} LC/MS [M+H] = 164 9; LC RT = 0.48 mm. (Column: BEH 08 2.1 x 50mm; Mobile Phase A: water with 0.05% TFA; Mobile Phase B: acetonitrile with 0.05% TFA; Temperature: 50 °C; Gradient: 2-98% B over 1.7 min; Flow: 0.8 mL/min).
References:
WO2019/213340,2019,A1 Location in patent:Page/Page column 108