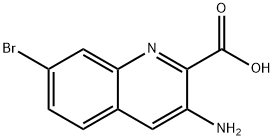
2-Quinolinecarboxylic acid, 3-amino-7-bromo- synthesis
- Product Name:2-Quinolinecarboxylic acid, 3-amino-7-bromo-
- CAS Number:1620239-25-9
- Molecular formula:C10H7BrN2O2
- Molecular Weight:267.08
1260807-99-5
37 suppliers
$124.00/250mg

1620239-25-9
6 suppliers
inquiry
Yield:-
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;water at 50; for 2 h;
Steps:
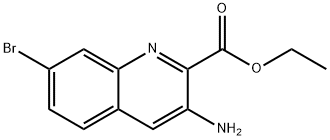
12.3 3-Amino-7-bromoquinoline-2-carboxylic acid
To a mixture of ethyl 3-amino-7-bromoquinoline-2-carboxylate (Ark Pharm, 113.9 mg, 0.3859 mmol) and lithium hydroxide monohydrate (82.8 mg, 1.97 mmol), THF (2.50 mL) was added, followed by water (0.50 mL). The resulting mixture was stirred at 50° C. for 2 h. The reaction mixture was then cooled to room temperature and water (25 mL) was then added, followed by AcOH (245.9 mg, 4.095 mmol) to adjust the pH to 5. The mixture was extracted with EtOAc (3*25 mL). The combined organic extract was washed with brine (50 mL), then dried over Na2SO4, and concentrated to yield a yellow solid (83.3 mg). The crude product was used directly in the next step without further purification. LCMS calc. for C10H8BrN2O2(M+H)+: m/z=267.0. found 267.0
References:
US2014/200216,2014,A1 Location in patent:Paragraph 0475; 0476