
2-(t-Butyldimethylsilyloxy)-6-bromonaphthalene synthesis
- Product Name:2-(t-Butyldimethylsilyloxy)-6-bromonaphthalene
- CAS Number:100751-65-3
- Molecular formula:C16H21BrOSi
- Molecular Weight:337.33
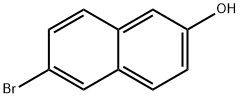
15231-91-1
392 suppliers
$7.00/5g

18162-48-6
668 suppliers
$9.00/5g

100751-65-3
21 suppliers
$75.00/1g
Yield:100751-65-3 100%
Reaction Conditions:
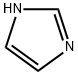
with 1H-imidazole in dichloromethane at 20; for 2 h;
Steps:
(6-bromonaphthalen-2-yloxy)(f?r*-butyl)dimethylsilane (7)TBSCl (347 mg, 2.3 mmol) was added to a solution of 6-bromonaphthalen-2-ol (446 mg, 2.0 mmol) in dichloromethane (20 ml) followed by the addition of imidazole (272 mg, 4.0 mmol). The reaction mixture was stirred at room temperature for 2 h and then water was added.
References:
WO2008/124812,2008,A1 Location in patent:Page/Page column 48-49
288-32-4
991 suppliers
$5.00/25g

15231-91-1
392 suppliers
$7.00/5g

100751-65-3
21 suppliers
$75.00/1g

288-32-4
991 suppliers
$5.00/25g

15231-91-1
392 suppliers
$7.00/5g

18162-48-6
668 suppliers
$9.00/5g

100751-65-3
21 suppliers
$75.00/1g